

Nitrohash. Here we are again with another article on nitrohash, a method for making hashish with liquid nitrogen that allows the trichomes to maintain all their qualities.

This time, we explain how to produce nitrohash with better accessories. In our 2010 issue 6, Soft Secrets introduced the Nitrohash technique, discovered by the great "hashman" Juanito Manoverde, a genuine resin expert whose travels all over the world have enabled him to master all possible techniques, traditional or modern. At the time, the nitrohash technique was just beginning to be developed, but now we are able to present a much more advanced system, although the basic principles remain the same. Juan Manoverde's inspiration for nitrohash began with liquid nitrogen in the kitchen. The main advantage of nitro-freezing is that foods maintain their properties - flavour, smell, vitamin contents - much better. Acquiring and storing nitrogen Handling and precautions Basic technique Leftover materials Assembly line Drying and preserving.
Cannabinoid and Terpenoid Reference Guide - Steep Hill Labs, Inc. ∆9-Tetrahydrocannabinol (THC) Formula: C21H30O2 Molecular Mass: 314.2246 g/mol Boiling Point: 157 °C (315 °F) Δ9-tetrahydrocannabinol (commonly referred to as “Δ9-THC,” “D9-THC,” “d9-THC” or simply “THC”) is a neutral cannabinoid, well known for being strongly psychoactive.
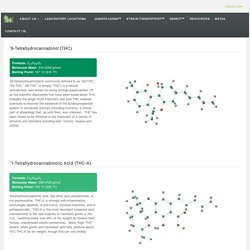
Of all the scientific discoveries that have been made about THC, probably the single most important was how THC enabled scientists to discover the existence of the Endocannabinoid system in vertebrate animals (including humans): a critical part of physiology that, up until then, was unknown. THC has been shown to be effective in the treatment of a variety of ailments and disorders including pain, tumors, nausea and ADHD. ∆1-Tetrahydrocannabinolic Acid (THC-A) Formula: C22H30O4 Molecular Mass: 358.4733 g/mol Boiling Point: 105 °C (220 °F) Tetrahydrocannabinolic acid, like other acid cannabinoids, is not psychoactive.
Cannabinol (CBN) Formula: C21H26O2 Molecular Mass: 310.1933 g/mol Boiling Point: 185 °C (365 °F) Linalool. Decarboxylation of cannabis: scientific info about temps and times. Decarboxylation of cannabis The following information about decarboxylation was not written by Cannabis Chris, but was pulled from another site during part of my research for “Marijuana Decarboxylation: how to decarboxylate medical marijuana” and “What is Decarboxylation?

“, as well as a future article with another method. This snippet about decarboxylation had too much good information for me to leave out. After reading this information, along with a graph that shows decarboxylation temperatures, I will be doing some experimenting with a few more methods of decarboxylating marijuana and I will post the results here. Anyway, here is some great information from one of the big pharma companies, about the decarboxylation process, some of the info is pretty boring, but please read it all. “The decarboxylation step may be carried out prior to or after extraction with liquid CO2. Preferably, decarboxylation is carried out in a multi-step heating process in which the plant material is: Like this: Extraction, Purification, and Isomerization of THC. Handbook of Cannabis - Google Books. Cannabinoid receptor antagonism increases female sexual motivation. The effects of progesterone (P) and the neurosteroid and P metabolite, 3alpha-hydroxy-5alpha-pregnan-20-one (3alpha,5alpha-THP) on ovariectomized (ovx), estradiol-3-benzoate (EB)-primed rats on sexual motivation, receptivity, and proceptivity were examined.
Changes in central P and 3alpha,5alpha-THP were measured following administration of EB, EB+P, EB+3alpha,5alpha-THP, or EB+inhibitor of 5alpha-reductase or P metabolism (epostane and finasteride)+P (Expt. 1). Partner preference was measured as the duration of time females in these different hormonal treatments spent in proximity to a male vs. female conspecific (Expt. 2). Receptivity (lordosis quotients and ratings) and proceptivity (darting, hopping, ear wiggling, and pacing), for different hormone treatments were assessed (Expt. 3 and Expt. 4, respectively). Conditioned place preference following hormone treatments and paced mating enabled assessment of sexual motivation (Expt. 5).