

A Brief Guide to Types of Organic Chemistry Formulae. Back to basics with today’s graphic, with a look at the different ways compounds in organic chemistry can be represented.

Obviously, if you’re a chemist, these will all be second nature, but as was quite fairly pointed out with regards to the food chemistry graphics, if you’re not well versed in chemistry, all of those lines and letters might well be a bit perplexing. Here’s a brief explanation of what they mean. The molecular formula of a compound gives the exact number of each different type of atom (i.e. type of element) present in one molecule of the compound.
This is useful because it allows you to work out the molecular weight of the compound, but can also be restrictive. It doesn’t give you any information about the bonds in the molecule, and, depending on how it’s written, might not even tell you about which functional groups are present. The empirical formula is the simplest ratio of the elements present in a molecule. Like this: Oxidations and reductions in organic chemistry: how do we recognize them? — Master Organic Chemistry. Oxidation And Reduction — Master Organic Chemistry. Here are two classes of reactions that are going to be very important going forward.
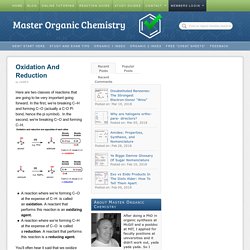
In the first, we’re breaking C–H and forming C–O (actually a C-O Pi bond, hence the pi symbol). In the second, we’re breaking C–O and forming C–H. BBC Bitesize - GCSE Chemistry - Polymers - Activity. Intermediate 2 Bitesize Chemistry - Plastics and Synthetic Fibres : Revision, Page 5. Flash. Conceptos de Quimica Organica Programas animados Para poderlos visualizar tiene que tener instalado el pugin de flash (Adobe)

Addition and Condensation Polymers and Polymerization. Pagina_14 — ocwus. Pagina_14 — ocwus. Practica de laboratorio - Ensayos - Angelitodulceme. PRACTICA DE LABORATORIO Comparación entre propiedades de sustancias iónicas, moleculares y atómicas.
Objetivos: *Distinguir fácilmente los tipos de enlace de la sal común , el azúcar y elmagnesio en base de sus propiedades. Hipótesis: Si comparamos cualitativamente el punto de fusión, solubilidad en agua y la conductividad eléctrica de la sal común, el azúcar y el magnesio, podemosdistinguir fácilmente los tipos de enlace químico que presentan cada una de ellas. Off the Shelf Chemistry. 1. The student will learn that chemicals are not something just found in laboratories. Our physical environment is composed of chemicals. Serendip.brynmawr.edu/sci_edu/waldron/pdf/WhoTookJerellsIpodTeachPrep.pdf.
Testing Foods for Organic Compounds. Today, I'd like to tell you about one of my favorite labs.

It is definitely "an oldie, but goodie" lab! There is nothing new, innovative, or earth-shattering about this lab. The idea has been around for as long as I have, and this is my 28th year in the classroom! My students always love this lab, and they often comment and share their surprise with me over the results they obtain. Two-products.jpg (1000×729) Google. Atom by Atom, Bond by Bond, a Chemical Reaction Caught in the Act. Alkenyl Functional Group. Carboxylic Acid Derivatives. Carboxylic Acid Derivatives A Substitution Reaction Which Starts With Addition Last time we completed our study of the reactions of aldehydes and ketones, compounds in which a carbonyl group is bonded either to carbons or hydrogens.

The typical reaction pattern for these compounds was addition, with a nucleophile adding to the carbonyl carbon and an electrophile adding to the carbonyl oxygen. Today we'll look at carboxylic acid derivatives. This group of compounds also contains a carbonyl group, but now there is an electronegative atom (oxygen, nitrogen, or a halogen) attached to the carbonyl carbon. This difference in structure leads to a major change in reactivity. Notice that each of these functional groups has either an oxygen, a nitrogen, or a halogen attached to the carbonyl carbon. WebQuests. What's the Organic Buzz?
Organic Chemistry in the Newsadapted from Fiona Clark's Webquest Memo. Www.adventuresinenergy.org/main.swf. Chemistry of Alcohol. Core Science: Assessment for Learning WebQuests. Higher Bitesize Chemistry - Reactions of carbon compounds : Revision. Carbon is 4 ever - Intro. As of July 1, 2013 ThinkQuest has been discontinued.

We would like to thank everyone for being a part of the ThinkQuest global community: Students - For your limitless creativity and innovation, which inspires us all. Teachers - For your passion in guiding students on their quest. Partners - For your unwavering support and evangelism. Parents - For supporting the use of technology not only as an instrument of learning, but as a means of creating knowledge.
We encourage everyone to continue to “Think, Create and Collaborate,” unleashing the power of technology to teach, share, and inspire. Best wishes, The Oracle Education Foundation. Lesson Plan: Chemistry Webquest for Mixture Separation in an Oil Refinery. 10.
What is the most common way to separate petroleum into various components? (The most common process is to use the different boiling temperatures of liquids to separate them.) _module02_int.pdf (application/pdf Objeto) Chapter21.pdf (application/pdf Objeto) Química Orgánica.