


Periodic Table Battleship I have posted a lot about Chemistry lately. My oldest has been studying it and really enjoying it. I love his science-y mind! Today I have a really fun & simple chemistry game to share. We played Periodic Table Battleship!
Practical Chemistry This website is for teachers of chemistry in schools and colleges. It is a collection of experiments that demonstrate a wide range of chemical concepts and processes. Some of the experiments can be used as starting-points for investigations or for enhancement activities. Many have links to carefully selected further reading and all include information and guidance for technicians. Chemistry is a practical science. Practical activities are not just motivational and fun: they also enable students to apply and extend their knowledge and understanding of chemistry in novel investigative situations, which can aid learning and memory and stimulate interest.
BioEd Online David Eagleman, PhD, author of the New York Times bestseller, Incognito: The Secret Lives of the Brain, provides an extraordinary look at the human brain and current neuroscience research. In this engaging series of videos, Dr. Eagleman explains the basics of brain function, and describes how this extremely complex, often misunderstood organ defines who we are. Dr. Resource: Everyday Exploration of Chemical Compounds InfoGraph Cards A resource blog, developed by a UK-based Newly Qualified Teacher, is gaining respectable notoriety with teachers and parents alike due to the accessibility of resource/revision cards (or can be printed out as posters) designed to help develop understanding of chemistry and chemical processes. Speaking to UKEdChat, the author of the resource said, “The site started off the back of a series of posters I made for my classroom to showcase the different groups of elements in the Periodic Table. A few people mentioned that they’d like copies, so I created the site in order to share the files for free. It pretty much escalated from there – a lot of people on twitter downloaded the files and used them in their own classrooms, and I also created a set of teaching versions of the elements graphics, with information missing, to be used in research tasks.” Since they proved popular, I continued making graphics on different facets of chemistry, as it’s a process I really enjoy anyway.
Nanoscience and nanotechnology - Joint study Royal Society and Royal Academy of Engineering Home . DESIGN SQUAD GLOBAL Come play again later! Come play again tomorrow! goREACT With goREACT, you can become a virtual chemist. Whether you're a novice or expert, the free play and guided modes make it fun and fascinating. - Initiate nearly 300 virtual chemical reactions by dragging elements into the Reaction Area.- Amazing images and videos illustrate the molecules you create.- Select alternate views of the Periodic Table to discover different aspects of the elements’ chemical properties.- Touch any of the Periodic Table's 118 elements to see an image and fun fact about it.- With helpful hints about reactions to try, there’s always something exciting to explore.- “Featured Reactions” menus guide you through themed sets of chemical reactions related to particular applications,such as the environment, beauty products or cars.- Learn more about how the Periodic Table is organized, and follow links to additional educational resources. Avec goREACT, vous pouvez devenir un chimiste virtuel.
100+ experiments in chemistry Chemistry videos - chemicum.com In order to make chemistry lessons more interesting, chemistry videos are being developed by Superaccu OÜ. Viewed >5000000 times. Some weirdest experiments: Gallium lives - remember Terminator II. There is a small alien hidden inside every mercury droplet. What is cold light or chemiluminescence. Creative Chemistry - Anodising Aluminium Student notes There are four stages, preparing the aluminium, preparing the anodising cell, anodising, and dyeing. Preparing the aluminium Using the scissors, cut out a piece of aluminium from your can. Watch out for sharp edges! About 5 cm x 2 cm is OK.
Some of The Best YouTube Channels for Physics and Chemistry Teachers July 11, 2015 Science in general is all about understanding how the world around us functions. It investigates the laws governing the internal workings of the universe and attempts to prove or disprove theories and assumption pertaining to it. For students, understanding scientific phenomena is integral to their overall intellectual and cognitive growth. It thrusts them into a world of exploration and problem solving and, most important of all, it unleashes their creativity and satiates their curious and probing minds. In today's post, we are sharing with you some excellent YouTube channels particularly curated for chemistry and physics teachers.
29jun_experiman The fourth competition, linked to the chat and video "Science: where can it take you?" is open from 29 May 2013 until 29 June 2013. This time the competition will be for teachers and for students. Teachers: are invited to submit a lessons plan (one lesson, or a sequence of lessons) using the video "Science: where can it take you?". Thus, the lesson plan should show how the video could be used in the classroom.
Database of Periodic Tables There are hundreds of periodic tables in web space, but there is only one comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Dr Mark R Leach. Harrington Periodic Tables So we start this effort tabula rasa (without preconceived ideas). 1) All atoms have a default "common denominator" structure at 270 mass units, irrespective of the element under discussion. 17 Science Experiments That Will Make Childhood Unforgettable We care for our children every day. But in 20 years it won’t be the everyday tasks we performed for them that they’ll remember. Instead, it will be the moments we spent together. Bright Side put together a selection of the 16 best science experiments that will both tear you away from your concerns and fascinate your children. They don’t require any special preparations or knowledge, but the satisfaction you and your kids will get from them will be high indeed.
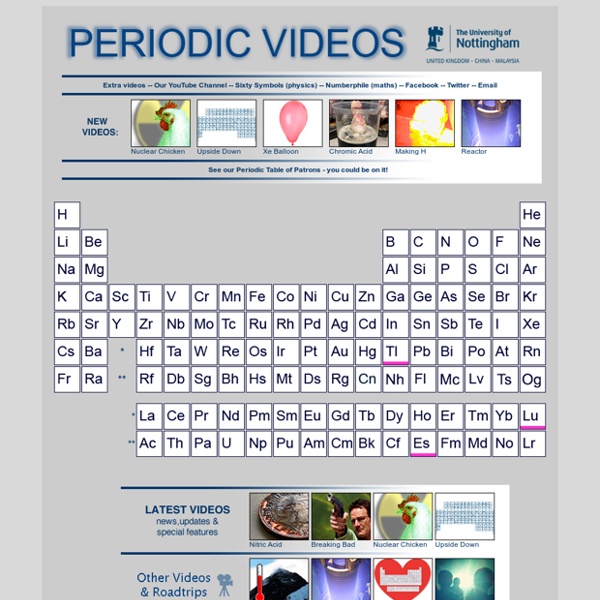
PTOV is created by video journalist Brady Haran working with chemists at The University of Nottingham. by mrpearl Mar 30