

CORE-Materials. Success fail About this capture CORE-Materials Collaborative Open Resource Environment – for Materials Resource Finder The CORE-Materials repository contains 1670 open educational resources (OERs) in Materials Science and Engineering, freely available under a range of Creative Commons licenses.
It also contains 132 copyrighted resources, which you might find useful. I'm looking for resources on leave blank to see all resources Click on the images to view resource details Latest News Resources on Novel Materials, including quasicrystals from AMES Lab Materials breakthrough - resources on graphene added Forming and Testing Techniques for Composite Materials Have you seen...? Continuum Mechanics video lectures from Imperial College London Animation of aluminium extrusion process Restoring a tooth video User comments "Thank you for the CC license, I used this for an article, on www.labgrab.com. "Thank you for help me to understand this technique. "WOW! Follow us on... 2010 University of Liverpool. Materials. V2.01 17-Jun-2004 1.
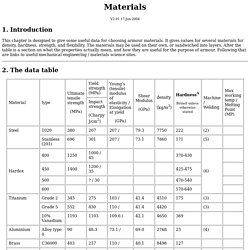
Introduction This chapter is designed to give some useful data for choosing armour materials. It gives values for several materials for density, hardness, strength, and flexibility. The materials may be used on their own, or sandwiched into layers. 2. Notes A. A description of the Rockwell Hardness Test is here.
Essential Documents. Assignment 1. Assignment 2. Assignment 3. Assignment 4. Assignment: Materials Testing. Further reading/ useful sites. Heat treatment of steel. Phase Diagrams Part 1. Phase Diagrams Part 2. 12.2: The Arrangement of Atoms in Crystalline Solids. Because a crystalline solid consists of repeating patterns of its components in three dimensions (a crystal lattice), we can represent the entire crystal by drawing the structure of the smallest identical units that, when stacked together, form the crystal.

This basic repeating unit is called a unit cell. For example, the unit cell of a sheet of identical postage stamps is a single stamp, and the unit cell of a stack of bricks is a single brick. In this section, we describe the arrangements of atoms in various unit cells. Unit cells are easiest to visualize in two dimensions. In many cases, more than one unit cell can be used to represent a given structure, as shown for the Escher drawing in the chapter opener and for a two-dimensional crystal lattice in Figure 12.2. The Unit Cell There are seven fundamentally different kinds of unit cells, which differ in the relative lengths of the edges and the angles between them (Figure 12.4).
Figure 12.5 The Three Kinds of Cubic Unit Cell. Summary. Main_page.