

P7C3. P7C3 is a drug related to latrepirdine (dimebon), which has neuroprotective and proneurogenic effects, and may be potentially useful for the treatment of Alzheimer's disease and similar neurodegenerative disorders.
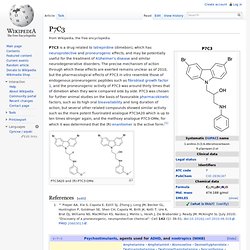
The precise mechanism of action through which these effects are exerted remains unclear as of 2010, but the pharmacological effects of P7C3 in vitro resemble those of endogenous proneurogenic peptides such as fibroblast growth factor 1, and the proneurogenic activity of P7C3 was around thirty times that of dimebon when they were compared side by side. P7C3 was chosen for further animal studies on the basis of favourable pharmacokinetic factors, such as its high oral bioavailability and long duration of action, but several other related compounds showed similar activity such as the more potent fluorinated analogue P7C3A20 which is up to ten times stronger again, and the methoxy analogue P7C3-OMe, for which it was determined that the (R) enantiomer is the active form.[1]
Cinnarizine. Pharmacokinetics[edit] A study that administered 75 mg doses of cinnarizine, twice a day for twelve days, to healthy volunteers, observed that cinnarizine did build up in the body, with a steady-state accumulation factor of 2.79 +/- 0.23.[9] However, the AUCT for this amount of time (T=12 days) was not significantly different from the AUC∞, which was estimated from the single dose administration.
As a very weakly basic and also lipophilic compound with low aqueous solubility, cinnarizine is able to cross the blood brain barrier by simple diffusion.[11][12] It is because of this property that it is able to exert its effects on cerebral blood flow in the brain.[13] Pharmacodynamics[edit] Treatment[edit] The vestibular system's semicircular canal – a cross-section. Cinnarizine has also been found to be a valuable second-line treatment for idiopathic urticarial vasculitis.[34] Side effects[edit] Side effects experienced while taking cinnarizine range from the mild to the quite severe.
HT-0712. HT-0712 also known as IPL-455903,[1] is an experimental cognitive enhancing drug (nootropic) which is currently undergoing clinical trials.
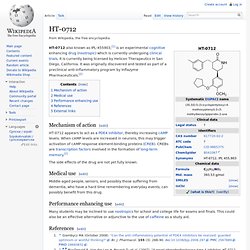
It is currently being licensed by Helicon Therapeutics in San Diego, California. It was originally discovered and tested as part of a preclinical anti-inflammatory program by Inflazyme Pharmaceuticals.[2] Mechanism of action[edit] HT-0712 appears to act as a PDE4 inhibitor, thereby increasing cAMP levels. When cAMP levels are increased in neurons, this may trigger activation of cAMP response element-binding proteins (CREB). The side effects of the drug are not yet fully known. Medical use[edit] Middle-aged people, seniors, and possibly those suffering from dementia, who have a hard time remembering everyday events, can possibly benefit from this drug. Performance enhancing use[edit] Many students may be inclined to use nootropics for school and college life for exams and finals. S-17092. S-17092 is a drug which acts as a selective inhibitor of the enzyme Prolyl endopeptidase.[1] This enzyme is involved in the metabolic breakdown of a number of neuropeptide neurotransmitters in the brain,[2][3] and so inhibiting the action of the enzyme increases the activity of these neuropeptides.
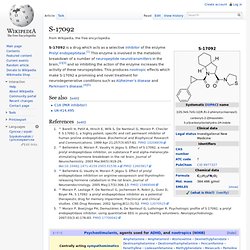
This produces nootropic effects which make S-17092 a promising and novel treatment for neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease.[4][5] See also[edit] References[edit] Jump up ^ Barelli H, Petit A, Hirsch E, Wilk S, De Nanteuil G, Morain P, Checler F. S 17092-1, a highly potent, specific and cell permeant inhibitor of human proline endopeptidase. PRL-8-53. Nootropic effects[edit] A single study in humans was reported in 1978.
The double-blind trial of PRL-8-53 in 47 healthy volunteers measured its effects on a variety of cognitive measures. 5 mg of the drug was administered orally 2–2.5 hours before the study tasks.[1] Subjects were given 12-item word lists to memorize as part of the trial. While initial word acquisition performance on PRL-8-53 was only 107.46% of baseline, subjects recalled words at 132.5–142.7% of the baseline rate 24 hours after testing, and at 145.2–146.2% after a week. Stronger effects were noted in the bottom 60% of subjects (who recalled 6 or fewer words on placebo at 24h), with 24 hour retention improved to 187.5–191% of baseline, and one week retention to 200–205%.