

Connectomics: Think small. Brains come in different sizes.
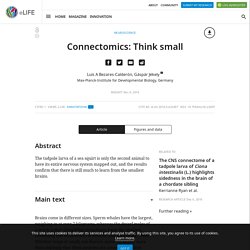
Sperm whales have the largest, weighing in at over 7 kilograms, whereas the dwarf males of a worm called Dinophilus gyrociliatus have the smallest, containing just 66 neurons (Windoffer and Westheide, 1988). Whether large or small, one feature unites all brains more than anything else: their neurons are connected to form intricate networks. This property is not seen in other organs, like the liver, kidneys or skin. Mapping networks of neurons has now become a central theme in neuroscience, as evidenced by the rise of the field of connectomics. Producing wiring diagrams of neural networks – or connectomes – that show the specific connections between cells requires use of electron microscopy to image ultrathin slices of neural tissue. Now, in eLife, Kerrianne Ryan, Zhiyuan Lu and Ian Meinertzhagen from Dalhousie University report that they have mapped the connectome of a tadpole larva of the ascidian Ciona intestinalis (Ryan et al., 2016).
Catecholamine. A catecholamine (/ˌkætəˈkoʊləmiːn/; CA) is a monoamine, an organic compound that has a catechol (benzene with two hydroxyl side groups at carbons 1 and 2) and a side-chain amine.[1] Catechol can be either a free molecule or a substituent of a larger molecule, where it represents a 1,2-dihydroxybenzene group.
Catecholamines are derived from the amino acid tyrosine, which is derived from dietary sources as well as synthesis from phenylalanine.[2] Catecholamines are water-soluble and are 50%-bound to plasma proteins in circulation. Included among catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine. SOX2. SRY (sex determining region Y)-box 2, also known as SOX2, is a transcription factor that is essential for maintaining self-renewal, or pluripotency, of undifferentiated embryonic stem cells.
Sox2 has a critical role in maintenance of embryonic and neural stem cells.[5] Sox2 holds great promise in research involving induced pluripotency, an emerging and very promising field of regenerative medicine.[6] Function[edit] Stem cell pluripotency[edit] LIF (Leukemia inhibitory factor) signaling, which maintains pluripotency in mouse embryonic stem cells, activates Sox2 downstream of the JAK-STAT signaling pathway and subsequent activation of Klf4 (a member of the family of Kruppel-like factors). NPM1, a transcriptional regulator involved in cell proliferation, individually forms complexes with Sox2, Oct4 and Nanog in embryonic stem cells.[8] These three pluripotency factors contribute to a complex molecular network that regulates a number of genes controlling pluripotency.
The Evolution and Function of Melanopsin in Craniates. Seritonin vs melatonin. Satellite glial cell. Satellite glial cells are glial cells that cover the surface of nerve cell bodies in sensory, sympathetic and parasympathetic ganglia.[1][2] Both satellite glial cells (SGCs) and Schwann cells (the cells that ensheathe some nerve fibers in the PNS) are derived from the neural crest of the embryo during development.[3] SGCs have been found to play a variety of roles, including control over the microenvironment of sympathetic ganglia.[2] They are thought to have a similar role to astrocytes in the central nervous system (CNS).[2] They supply nutrients to the surrounding neurons and also have some structural function.
Satellite cells also act as protective, cushioning cells. Organogenesis (Fetal Development) All neurons and supporting cells are derived from ectoderm.

Organogenesis (Fetal Development) WikiGenes - Lhx9 - LIM homeobox protein 9. Pallium (neuroanatomy) Importantly, the lateral and ventral parts of the pallium produce also deep to their respective sectors of subpial olfactory cortex sets of pallial nuclei, the neurons entering the claustrum, rostrally, and the pallial amygdala, caudally.
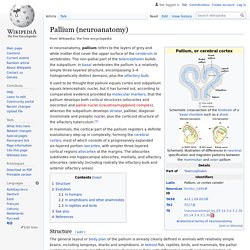
The concept of hypopallium refers to this histogenetically unitary complex of olfactory (piriform) cortex and deep pallial nuclei. In reptiles and birds the hypopallium becomes differentially enlarged (largest in crocodiles and birds, whose olfactory cortex gets nevertheless reduced), whereas in mammals it becomes reduced to the claustroamygdaloid complex and relatively enlarged olfactory (prepiriform and piriform) cortex. Radial glial cell. Radial glial cells are bipolar-shaped cells that span the width of the cortex in the developing vertebrate central nervous system (CNS)[2][3] and serve as primary progenitor cells capable of generating neurons, astrocytes, and oligodendrocytes.[4] Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.
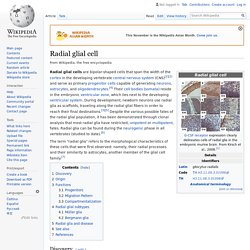
During development, newborn neurons use radial glia as scaffolds, traveling along the radial glial fibers in order to reach their final destinations.[3][5] Despite the various possible fates of the radial glial population, it has been demonstrated through clonal analysis that most radial glia have restricted, unipotent or multipotent, fates. Radial glia can be found during the neurogenic phase in all vertebrates (studied to date).[6] Endothelium - Embryonic Development & Stem Cells - LifeMap Discovery. Gap junction - Wikipedia. A gap junction may also be called a nexus or macula communicans.
When found in neurons or nerves it may also be called an electrical synapse. While an ephapse has some similarities to a gap junction, by modern definition the two are different. One gap junction channel is composed of two connexons (or hemichannels), which connect across the intercellular space.[4][5][6] Gap junctions are analogous to the plasmodesmata that join plant cells.[7] Gap junctions occur in virtually all tissues of the body, with the exception of adult fully developed skeletal muscle and mobile cell types such as sperm or erythrocytes. Gap junctions, however, are not found in simpler organisms such as sponges and slime molds. Structure[edit] L-DOPA - Wikipedia. Medical use[edit] In addition, L-DOPA, co-administered with a peripheral DDCI, has been investigated as a potential treatment for restless leg syndrome.
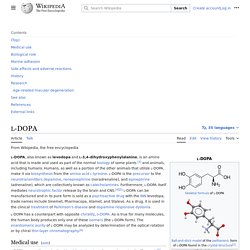
However, studies have demonstrated "no clear picture of reduced symptoms".[8] Neuromelanin - Wikipedia. 5,6-dihydroxyindole, the monomer out of which neuromelanin polymers are formed.
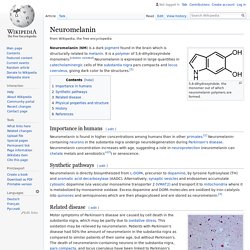
Neuromelanin (NM) is a dark pigment found in the brain which is structurally related to melanin. It is a polymer of 5,6-dihydroxyindole monomers. Meningeal lymphatic vessels - Wikipedia. Cardiac neural crest complex - Wikipedia. The cardiac neural crest complex is a form of neural crest.[1] Derivation and migration[edit] Enteric nervous system - Wikipedia. Truncal neural crest - Wikipedia. The truncal neural crest or trunk neural crest is a form of neural crest.[1] The trunk neural crest lies between the vagal and sacral neural crest and gives rise to two groups of cells.
One group migrates dorsolateral and populates the skin, forming pigment cells and the other migrates ventrolateral through the anterior sclerotome to become the epinephrine-producing cells of the adrenal gland and the neurons of the sympathetic nervous system. Cranial neural crest - Wikipedia. File:CG Heart.gif.