

Water risk as world warms. Dieter Telemans/Panos Water scarcity in parts of Africa could become worse, according to a complementary set of climate projections.

When pondering the best way to study the impact of climate change, researcher Hans Joachim Schellnhuber liked to recall an old Hindu fable. Six men, all blind but thirsty for knowledge, examine an elephant. One fumbles the pachyderm’s sturdy side, while others grasp at its tusk, trunk, knee, ear or tail. Global warming. Global mean land-ocean temperature change from 1880 to 2014, relative to the 1951–1980 mean.

The black line is the annual mean and the red line is the 5-year running mean. The green bars show uncertainty estimates. Source: NASA GISS. IPCC - Intergovernmental Panel on Climate Change. Science: search results. Greenhouse Gases. IntroductionWater VaporCO2CH4OzoneN2OCFCsCOAdditional Information Introduction What are greenhouse gases?

Many chemical compounds present in Earth's atmosphere behave as 'greenhouse gases'. These are gases which allow direct sunlight (relative shortwave energy) to reach the Earth's surface unimpeded. Halocarbon. Ozone. Ozone /ˈoʊzoʊn/ (systematically named 1λ1,3λ1-trioxidane and μ-oxidodioxygen), or trioxygen, is an inorganic molecule with the chemical formula O 3(μ-O) (also written [O(μ-O)O] or O 3).
It is a pale blue gas with a distinctively pungent smell. Ozone layer. Nitrous oxide. Nitrous oxide, commonly known as laughing gas, nitrous, nitro, or NOS[1] is a chemical compound with the formula N 2O.
It is an oxide of nitrogen. At room temperature, it is a colourless, non-flammable gas, with a slightly sweet odour and taste. It is used in surgery and dentistry for its anaesthetic and analgesic effects. It is known as "laughing gas" due to the euphoric effects of inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is also used as an oxidizer in rocketry and in motor racing to increase the power output of engines.
Nitrous oxide gives rise to NO (nitric oxide) on reaction with oxygen atoms, and this NO in turn reacts with ozone. Methane. Arctic Methane Bubbles. Scientists are continually working to improve estimates of just how much methane, a potent greenhouse gas, is being emitted from the Arctic.
A new study led by researcher Natalia Shakhova of the University of Alaska, Fairbanks, and the Russian Academy of Sciences' Far Eastern Branch reports that methane releases from one part of the Arctic Ocean are more than twice what scientists previously thought. Shakhova and her colleagues investigated releases of methane from permafrost underneath a shallow part of the Arctic Ocean called the East Siberian Arctic Shelf, which sits in the ocean north of Siberia and east of the Lena River Delta.
There, the underwater permafrost serves as a cap over methane in the seafloor. The permafrost is thawing, though and losing its ability to hold in the methane. "Thus, methane could release in large amounts," Shakhova said. Threat of ‘Methane Time Bomb. 30 Oct 2008: Report by susan q. stranahan For the past 15 years, scientists from Russia and other nations have ventured into the ice-bound and little-studied Arctic Ocean above Siberia to monitor the temperature and chemistry of the sea, including levels of methane, a potent greenhouse gas.

Their scientific cruises on the shallow continental shelf occurred as sea ice in the Arctic Ocean was rapidly melting and as northern Siberia was earning the distinction — along with the North American Arctic and the western Antarctic Peninsula —of warming faster than any place on Earth. Until 2003, concentrations of methane had remained relatively stable in the Arctic Ocean and the atmosphere north of Siberia.
But then they began to rise. Methane Emissions. Methane (CH4) is the second most prevalent greenhouse gas emitted in the United States from human activities.
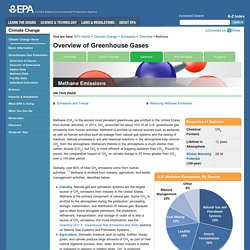
In 2013, CH4 accounted for about 10% of all U.S. greenhouse gas emissions from human activities. Methane is emitted by natural sources such as wetlands, as well as human activities such as leakage from natural gas systems and the raising of livestock. Natural processes in soil and chemical reactions in the atmosphere help remove CH4 from the atmosphere. Methane's lifetime in the atmosphere is much shorter than carbon dioxide (CO2), but CH4 is more efficient at trapping radiation than CO2.
Pound for pound, the comparative impact of CH4 on climate change is 25 times greater than CO2 over a 100-year period. Globally, over 60% of total CH4 emissions come from human activities. [1] Methane is emitted from industry, agriculture, and waste management activities, described below. Industry. Carbon dioxide. Carbon dioxide (chemical formula CO2) is a naturally occurring chemical compound composed of 2 oxygen atoms each covalently double bonded to a single carbon atom.
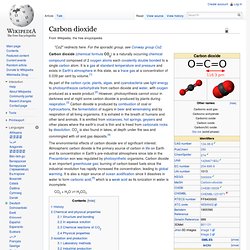
It is a gas at standard temperature and pressure and exists in Earth's atmosphere in this state, as a trace gas at a concentration of 0.039 per cent by volume.[1] GreenHouse Gas Online. Most potent greenhouse gas detected. Ted talks about global issues. Climate Reality. The Great Global Warming Swindle. [First published on Google Knol on September 20th, 2008] Summary There are scientists who dispute the popular belief about the cause of global warming, also known as climate change.
They have identified five reasons why man-made production of carbon dioxide cannot cause global warming. Climate Change.