

Salters GCE AS Chemistry past papers B Advanced Subsidiary syllabus-specification H035, past papers practice exam links, Module Unit F331 Chemistry of Life, EL Elements of Life, DF Developing Fuels, Module Unit F332 Chemistry of Natural Resources, ES Elem. Doc Brown's Advanced Level Chemistry updated November 26th 2012 GCE Salters Advanced Subsidiary AS Chemistry HO35 Syllabus-specification A2 Chemistry Unit EL Elements of Life * DF Developing Fuels * ES Elements from the Sea * A The Atmosphere * PR Polymer Revolution * CI = Chemical Ideas textbook reference for My unofficial support for this OCR GCE Salters Chemistry Advanced Subsidiary AS Chemistry syllabus-specification.

Scroll down and each module, topic or unit of the specification is linked to potentially useful sections on this site[Each link opens up in a new window] [PAST PAPERS] [ALL LINKS SHOULD WORK IF THIS PAGE IS SAVED] Tyler DeWitt. Microscale Chemistry - The microscale synthesis of aspirin- Learn Chemistry. Nuffield Advanced Chemistry - Investigations involving rates of reaction. Decomposition of Hydrogen Peroxide. Starters for Ten- Learn Chemistry. Chemistry - few periodic rules to rule them all :) Creative Chemistry AS and A Level Stuff. Doc Brown's Advanced Level Chemistry Revision Index A Level AS A2 IB level student exam revision help AQA Edexcel OCR Salters WJEC CCEA.
Level Chemistry Revision. The Periodic Table of Videos - University of Nottingham. How to calculate the Empirical Formula. Atomicmusicalchairs. Salters Advanced Chemistry, Educational Studies. Welcome to Salters’ Advanced Chemistry website.
This Advanced level chemistry course, developed at the Science Education Group in the University of York, offers an exciting, context based approach to studying chemistry. The course is currently followed by over 17000 students across the United Kingdom. We work closely with OCR (the examination board) who offer dedicated assessment for the course. The website is currently under construction and pages will be regularly added over the next few months.
New Resources A series of exciting new resources have been added to to the DIY page to support teaching and learning of SAC. Are you a school science technician? Do you want to increase your confidence when making up solutions? Hands-on chemical handling and preparation of solutions course 2nd and 3rd July 2014 This three session course will offer you hands-on experience where you will learn to prepare solutions used in school science pratical work. The Essential Chemical Industry - online. Extraction of iron on a match head! Students reduce iron(III) oxide with carbon on a match head to produce iron in this small scale example of metal extraction.
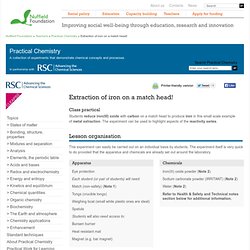
The experiment can be used to highlight aspects of the reactivity series. This experiment can easily be carried out on an individual basis by students. The experiment itself is very quick to do provided that the apparatus and chemicals are already set out around the laboratory. Iron(III) oxide powder (Note 2) Sodium carbonate powder (IRRITANT) (Note 2) Water (Note 2) Refer to Health & Safety and Technical notes section below for additional information. Eye protection. Chemistry-compounds-iron oxide and aluminium. Nitrogen does diamond. A top view of the calculated nitrogen polymorph structure (left) and the diamond-esque N10 structure (right) © APS Nitrogen will form an unusual cage-like structure when subjected to high pressures, an international team of researchers has calculated.
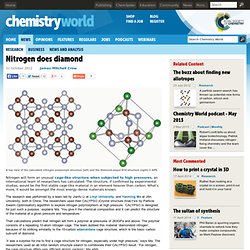
The structure, if confirmed by experimental studies, would be the first stable cage-like material in an element heavier than carbon. What’s more, it would be amongst the most energy-dense materials known. The research was performed by a team led by Jianfu Li at Linyi University, and Yanming Ma at Jilin University, both in China. The researchers used their CALYPSO (Crystal structure AnaLYsis by Particle Swarm Optimisation) algorithm to explore nitrogen polymorphism at high pressure. Their calculations predict that nitrogen will form a polymer at pressures of 263GPa and above. ‘It was a surprise for me to find a cage structure for nitrogen, especially under high pressure,’ says Ma. Calcium acetate. Production[edit] Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as limestone or marble) in vinegar: CaCO3 + 2CH3COOH → Ca(CH3COO)2 + H2O + CO2 Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Uses[edit] In kidney disease, blood levels of phosphate may rise (called hyperphosphatemia) leading to bone problems. References[edit]