

Photoelectric Effect – The Physics Hypertextbook. Dilemma Under the right circumstances light can be used to push electrons, freeing them from the surface of a solid.

This process is called the photoelectric effect (or photoelectric emission or photoemission), a material that can exhibit this phenomena is said to be photoemissive, and the ejected electrons are called photoelectrons; but there is nothing that would distinguish them from other electrons. All electrons are identical to one another in mass, charge, spin, and magnetic moment. The photoelectric effect was first observed in 1887 by Heinrich Hertz (1857–1894) during experiments with a spark-gap generator (the earliest form of radio receiver). In these experiments, a spark is generated between two small metal spheres in the transmitter to induce a similar spark to jump between two different metal spheres in the receiver. While this is interesting, it is hardly amazing. Knocking electrons free from the photoemissive plate would give it a slight positive charge. New idea. pH Calculator - Calculates pH of a Solution. Chemical of the Week. Have you ever been to an aerial fireworks show at an amusement park, baseball game, Fourth of July celebration, or on New Year's Eve and wondered about how all the impressive colors and sounds are produced?

People everywhere enjoy the fantastic explosions and the brilliant light displays of fireworks. However, these spectacles are much more than just a form of entertainment. Each firework launched into the sky is a precisely formed assembly of chemicals and fuel, carefully calibrated to produce a particular effect – a red chrysanthemum spray accompanied by a powerful explosion, or a blue strobe, for example. Understanding how the contents of a firework produce the impressive variety of colors, forms, and sound intensities requires only a simple understanding of chemical reactions. Fireworks generate three very noticeable forms of energy: a tremendous release of sound, bright light, and heat. A firework is ignited by lighting the main fuse. Chemistry of Fireworks Firework Safety. Alpha Widgets: "Lewis structure" - Free Chemistry Widget.
ChemWiki: The Dynamic Chemistry E-textbook - Chemwiki. The PubChem Project. Chem 152. Chemistry 152.

General Chemistry II These files are provided for students in Chemistry 152 lecture at Pima Community College for the 2012-2013 academic year. These are pdf files and require Adobe Acrobat Reader (downloaded free from the Adobe web site) Lecture Information: CHM 152IN Syllabus for Spring 2013 CHM 152 Topic List for students using the textbook by Kotz, John C., Treichel, Paul M., and Townsend, John R., Chemistry & Chemical Reactivity, 7th Ed., Thomson-Brooks/Cole, 2009 Laboratory Information: CHM 152IN Laboratory Schedule for Spring 2013 This laboratory schedule contains the schedule for students using the General Chemistry in Action laboratory manual, 2nd Edition by Thomas Selegue and David A. Laboratory Notebooks and Laboratory Reports. Chemistry 223 - Chapter Guides - How To Study For the A.C.S. Exam. Here are a few hints on how to study for the American Chemical Society (A.C.S.)

Final Exam in CH 223. First, the basics: The A.C.S. Exam is comprehensive, covering Chapters 1 through 23 in our text. There will be 75 multiple choice questions on the exam. Each multiple choice question will have four possible answers. There is one important handout for you to view before the exam: The A.C.S. The A.C.S. It is important to remember that you have made it this far in chemistry through your hard work and refined study habits, and they will help you during the exam... even if you don't know that they are there, they'll come back to help you! Here are some links to various study materials from CH 221, CH 222 and CH 223.
Be sure to check out the Simulation Page which lists all of the drills, tutorials, etc. to help you learn the various topics we've covered throughout the year... they might prove useful as you remember what you already know. Chemistry Review Activities. I re-organized the course during the 2014 - 2015 school year.
Some review activities were moved to new units. This has resulted in a change to some of the file names, so direct links to the individual activities may need to be changed. These are not graded assignments. They are intended only as practice of concepts and vocabulary that are essential to your success in this course. Most of these interactive review activities work equally well in recent versions of Internet Explorer, Firefox, Google Chrome, and Safari.
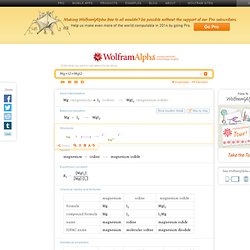
Thermodynamics. Chemistry Learning Center Tutorials. General GUIDES. Redox. Acids and bases. Titrations. Equilibrium. Greenhouse Gases and Paleoclimatology - Project. Rate. Random. Solution. Bonding/Nonbonding. Alpha: Computational Knowledge Engine. Mg+I2>MgI2. Support Wolfram|Alpha Wolfram Alpha Pro » x Image InputX Upload an image from your computer: Enter a URL for an image: Terms | Privacy All images »Recent images Loading...
Sample images Data InputX Give numeric, tabular, or other data. Save for Later Use Use without Saving Upload a file »Terms | Privacy All data »Recent data Sample data categories-currency dates-categories countries-currency-numbers.
Periodic Table. Tips. Experiments. Stoichiometry. Gas laws. Organic chemistry. Fundamentals. Reactions.