

Hydrogen Peroxide Health & Safety Tips. What is Hydrogen Peroxide?
Hydrogen peroxide was first discovered in the early 19th century, but the pure chemical was not produced until 1894. Today, this chemical is produced in quantities of more than 2 million tons worldwide each year. Hydrogen peroxide is a chemical compound usually produced as a diluted solution. This colorless, slightly viscous chemical has a somewhat sharp odor. Inhibition of peroxidase and catalase activities and modulation of hydrogen peroxide level by inositol phosphoglycan-like compounds.
Inositol phosphoglycan-like compounds are produced by the hydrolysis of the membrane bound glycosyl phosphoinositides.

Besides being short term mediators of insulin action, they inhibit peroxidases and catalase, increasing the concentration of cellular hydrogen peroxide. Although high concentrations of hydrogen peroxide are toxic, moderate increases of its basal level are signals for different metabolic pathways. The inhibitor, localized in the cytosol of the cell, acts on peroxidases and catalase of the same tissue (homologous action) and of other tissues or organisms (heterologous action). The inositol phosphoglycan-like compound inhibits peroxidases with different prosthetic groups, i.e. containing iron such as: thyroid peroxidase, lactoperoxidase, horseradish peroxidase, soy bean peroxidase; and containing selenium such as glutathione peroxidase and 2-cys peroxiredoxin with no prosthetic group.
Effect of Dietary Vitamin C and Catalase Inhibition on Antioxidants and Molecular Markers of Oxidative Damage in Guinea Pigs: Free Radical Research: Vol 21, No 2. (IUCr) The first structure of a cold-active catalase from <span class="it"><i>Vibrio salmonicida</i></span> at 1.96 Å reveals structural aspects of cold adaptation. Catalase evolved to concentrate H2O2 at its active site. Catalase is a homo-tetrameric enzyme that has its heme active site deeply buried inside the protein.

Its only substrate, hydrogen peroxide (H2O2), reaches the heme through a 45 A-long channel. Large-subunit catalases, but not small-subunit catalases, have a loop (gate loop) that interrupts the major channel. Two accesses lead to a gate that opens the final section of the channel to the heme; gates from the R-related subunits are interconnected. Using molecular dynamic simulations of the Neurospora crassa catalase-1 tetramer in a box of water (48,600 molecules) or 6M H2O2, it is shown that the number of H2O2 molecules augments at the surface of the protein and in the accesses to the gate and the final section of the channel. Heme - Definition, Structure and Function. Heme Definition A heme is an organic, ring-shaped molecule.
Due to its special structure, a heme is capable of holding, or “hosting” an iron molecule. A heme is made from 4 pyrroles, which are small pentagon-shaped molecules made from 4 carbons and 1 nitrogen. Substrate flow in catalases deduced from the crystal structures of active site variants of HPII from Escherichia coli. The active site of heme catalases is buried deep inside a structurally highly conserved homotetramer.
Channels leading to the active site have been identified as potential routes for substrate flow and product release, although evidence in support of this model is limited. To investigate further the role of protein structure and molecular channels in catalysis, the crystal structures of four active site variants of catalase HPII from Escherichia coli (His128Ala, His128Asn, Asn201Ala, and Asn201His) have been determined at approximately 2.0-A resolution. The solvent organization shows major rearrangements with respect to native HPII, not only in the vicinity of the replaced residues but also in the main molecular channel leading to the heme distal pocket. In the two inactive His128 variants, continuous chains of hydrogen bonded water molecules extend from the molecular surface to the heme distal pocket filling the main channel. Catalase - Proteopedia, life in 3D. LoadScript /wiki/extensions/jsmol/j2s/core/package.js loadScript /wiki/extensions/jsmol/j2s/core/corejmol.z.js loadScript /wiki/extensions/jsmol/j2s/J/translation/PO.js loadScript /wiki/extensions/jsmol/j2s/core/corescript.z.js.
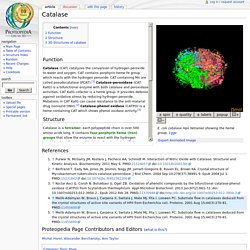
Tried and true but something new: analyzing the enzymatic activity of catalase: Journal of Biological Education: Vol 54, No 5. Catalase and hydrogen peroxide diagram. Inhibitors of Catalase Reaction. Diversity of structures and properties among catalases. Catalase Structure Tutorial Text. Elizabeth M.

Boon '97, Aaron Downs '00, and David Marcey Contents: Crystallization of Animal Catalase and Studies on its Optimum Temperature.