

Erratum: Vol. 52, No. SS-1. Persons using assistive technology might not be able to fully access information in this file.

For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. On page 7 in the CDC's Surveillance Summaries, "Surveillance for Safety After Immunization: Vaccine Adverse Event Reporting System (VAERS)---United States, 1991--2001," published on January 24, 2003, an error occurred in the last sentence of the first paragraph. The sentence should read, "On February 25, 2002, the manufacturer withdrew the vaccine from the market, citing poor sales. " On page 23, errors occurred in Figures 5 and 7. Figure 5 Return to top.Figure 7 Return to top. Adenoviridae. They have a broad range of vertebrate hosts; in humans, more than 50 distinct adenoviral serotypes have been found to cause a wide range of illnesses, from mild respiratory infections in young children (known as the common cold) to life-threatening multi-organ disease in people with a weakened immune system.[1] Virology[edit] Classification[edit] This family contains the following genera:[1] Diversity[edit] Classification of Adenoviridae can be complex.
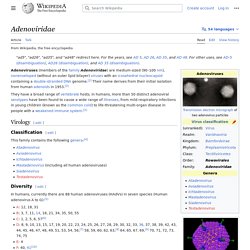
In humans, currently there are 88 human adenoviruses (HAdVs) in seven species (Human adenovirus A to G):[3] Different types/serotypes are associated with different conditions:[9] respiratory disease (mainly species HAdV-B and C)conjunctivitis (HAdV-B and D)gastroenteritis (HAdV-F types 40, 41, HAdV-G type 52)obesity or adipogenesis (HAdV-A type 31, HAdV-C type 5, HAdV-D types 9, 36, 37) [10] The types are called Human mastadenovirus A–G by the ICTV, as all are members of Mastadenovirus. Structure[edit] Genome[edit] Adaptive immune system. Subsystem of the immune system that is composed of specialized, systemic cells and processes Google Ngram of "acquired immunity " vs.
"adaptive immunity". The peak for "adaptive" in the 1960s reflects its introduction to immunology by Robert A. Good and use by colleagues; the explosive increase in the 1990s was correlated with the use of the phrase "innate immunity". Like the innate system, the adaptive immune system includes both humoral immunity components and cell-mediated immunity components and destroys invading pathogens. Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to future encounters with that pathogen. The cells that carry out the adaptive immune response are white blood cells known as lymphocytes. Adverse Effects of Vaccines: Evidence and Causality. IOM. 2001a.

Immunization safety review: Measles-mumps-rubella vaccine and autism. Washington, DC: National Academy Press. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Adverse events, events not previously reported in the medical literature or demonstrated in epidemiologic studies?

Surveillance for Safety After Immunization: Vaccine Adverse Event Reporting System (VAERS) Persons using assistive technology might not be able to fully access information in this file.

For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Please note: An erratum has been published for this article. To view the erratum, please click here. Electronic Support for Public Health - Vaccine Adverse Event Reporting System (ESP:VAERS) (Massachusetts) Why Did the CDC Silence the Million Dollar Harvard Project Charged With Upgrading Our Vaccine Safety Surveillance System? - Truth Snitch. 8 Things Your Doctor Didn't Tell You About the Flu Shot - Truth Snitch. An aluminium adjuvant in a vaccine is an acute exposure to aluminium. E.

Shardlow, M. Mold, C. The dangers of DNA vaccination. To the editor—DNA vaccination has entered clinical trials, and may ultimately be used on a large scale.

It involves the injection of naked DNA that encodes antigens under the control of a eukaryotic promoter, and results in strong and sustained humoral and cell–mediated responses. The generic nature of the technology will facilitate the development of new vaccines; DNA vaccines are stable and will not require continuous cold storage. These properties are likely to lead to extremely widespread clinical and agricultural use in both the developed and developing world. In the developing world, inadequate health care resources will make post–vaccination control difficult. Vaccine controverse. Lymph System. An Invisible Quantum Dot 'Tattoo' Could Be Used to ID Vaccinated Kids. New ID2020 Project to Build Biometric ID Program Around Infant Immunization.
Trust Stamp integrating biometric hash solution with Mastercard on children’s vaccine record system. Digital identity capabilities from Trust Stamp are now being integrated with Mastercard’s Wellness Pass solution, which it will launch in cooperation with Gavi in West Africa.
Proving identity without revealing any information about it is the idea behind Trust Stamp’s zero knowledge approach to online identity verification, according to a profile by Mastercard. The U.S. and Gavi, the Vaccine Alliance. Key Facts Gavi, the Vaccine Alliance (Gavi) is an independent public-private partnership and multilateral funding mechanism aims to “save lives, reduce poverty, and protect the world against the threat of epidemics.”Since its launch in 2000 through 2018, Gavi disbursed over $13 billion in support of immunization efforts in 76 low- and middle-income countries.
Gavi’s next replenishment round began in August 2019 and culminates in June 2020, before Gavi’s next five-year funding cycle begins.The U.S. government (U.S.) has supported Gavi since its creation through direct financial contributions, participation in Gavi’s governance, and technical assistance.The U.S. is one of Gavi’s top government donors. CAM bam ma'am. MEDICO. MedOmorpheses. Novel Coronavirus Pandemic Proportions. Signs Signal. Healthy Patterns.