

Thiol. Thiol with a blue-highlighted sulfhydryl group.
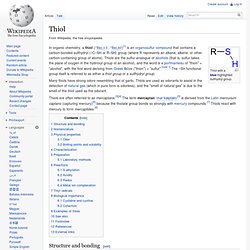
In organic chemistry, a thiol (/ˈθaɪˌɔːl/, /ˈθaɪˌɒl/)[1] is an organosulfur compound that contains a carbon-bonded sulfhydryl (–C–SH or R–SH) group (where R represents an alkane, alkene, or other carbon-containing group of atoms). Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl group of an alcohol), and the word is a portmanteau of "thion" + "alcohol", with the first word deriving from Greek θεῖον ("thion") = "sulfur".
[note 1] The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group. Many thiols have strong odors resembling that of garlic. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Carbonate. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverages — either by the addition of carbon dioxide gas under pressure, or by dissolving carbonate or bicarbonate salts into the water.
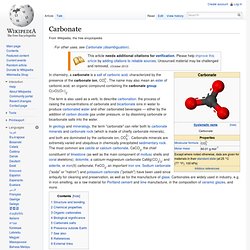
Structure and bonding[edit] The Lewis structure of the carbonate ion has two (long) single bonds to negative oxygen atoms, and one short double bond to a neutral oxygen This resonance can be summarized by a model with fractional bonds and delocalized charges: Chemical properties[edit] Metal carbonates generally decompose on heating, liberating carbon dioxide from the long term carbon cycle to the short term carbon cycle and leaving behind an oxide of the metal. A carbonate salt forms when a positively charged ion, M+, attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound:
Lexan. The chemical structure of Polycarbonate In this kinetic energy weapon test, a seven gram Lexan projectile was fired from a light gas gun at a velocity of 7,000 m/s (23,000 ft/s) at a cast aluminum block.
Lexan is a registered trademark for SABIC Innovative Plastics' (formerly General Electric Plastics) brand of a thermoplastic polycarbonate. It is also used in Textiles Machinery for High temperature and High Strength Polymer application. Development and patent[edit] Both companies applied for U.S. patents in 1955. History[edit] In the 1960s, NASA used Lexan-brand polycarbonate for astronaut helmet assemblies and visors which became known as "bubble helmets", including those used by the Apollo moon astronauts. Polycarbonate. Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures.
Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Polysulfone. Polysulfone repeating unit.
Polysulfone describes a family of thermoplastic polymers. These polymers are known for their toughness and stability at high temperatures. They contain the subunit aryl-SO2-aryl, the defining feature of which is the sulfone group. Polysulfones were introduced in 1965 by Union Carbide. Polystyrene. Styrene-butadiene. Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite[1]).
These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. [2] About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery.[3] SBR is not to be confused with a thermoplastic elastomer made from the same monomers, styrene-butadiene block copolymer. Acrylic acid. Production[edit] Acrylic acid is produced from propene which is a byproduct of ethylene and gasoline production.
Because acrylic acid and its esters have long been valued commercially, many other methods have been developed but most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene ("Reppe chemistry"): This method requires nickel carbonyl and high pressures of carbon monoxide. It was once manufactured by the hydrolysis of acrylonitrile which is derived from propene by ammoxidation, but was abandoned because the method cogenerates ammonium derivatives. Polyacrylic acid. Poly(acrylic acid) (PAA or Carbomer) is generic name for synthetic high molecular weight polymers of acrylic acid.
They may be homopolymers of acrylic acid, crosslinked with an allyl ether pentaerythritol, allyl ether of sucrose or allyl ether of propylene. In a water solution at neutral pH, PAA is an anionic polymer, i.e. many of the side chains of PAA will lose their protons and acquire a negative charge. This makes PAAs polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. Dry PAAs are found in the market as white and fluffy powders. Acrylonitrile. Acrylonitrile is a chemical compound with the formula C 3H 3N.
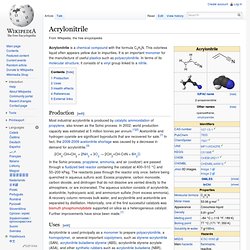
This colorless liquid often appears yellow due to impurities. Polyacrylonitrile. Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (C3H3N)n.
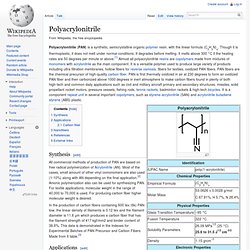
Though it is thermoplastic, it does not melt under normal conditions. It degrades before melting. It melts above 300 °C if the heating rates are 50 degrees per minute or above.[1] Almost all polyacrylonitrile resins are copolymers made from mixtures of monomers with acrylonitrile as the main component. Vinyl alcohol. Vinyl alcohol, also called ethenol (IUPAC name), is an alcohol. It is not to be confused with the drinking alcohol, ethanol. With the formula CH2CHOH, vinyl alcohol is an isomer of acetaldehyde and ethylene oxide.
Under normal conditions, it converts (tautomerizes) to acetaldehyde: Polyvinyl alcohol. Polyvinyl alcohol (PVOH, PVA, or PVAl) is a water-soluble synthetic polymer (not to be confused with polyvinyl acetate, a popular wood glue). It has the idealized formula [CH2CH(OH)]n. Polypropylene. Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging and labeling, textiles (e.g., ropes, thermal underwear and carpets), stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes. An addition polymer made from the monomer propylene, it is rugged and unusually resistant to many chemical solvents, bases and acids.
In 2008, the global market for polypropylene had a volume of 45.1 million metric tons, which led to a turnover of about $65 billion (~ €47.4 billion).[1] Chemical and physical properties[edit] Degradation[edit] Polypropylene is liable to chain degradation from exposure to heat and UV radiation such as that present in sunlight. History[edit] Polypropylene is the second most important plastic with revenues expected to exceed US$145 billion by 2019. Polypropylene. Polypropylene is one of those rather versatile polymers out there. It serves double duty, both as a plastic and as a fiber. As a plastic it's used to make things like dishwasher-safe food containers.
It can do this because it doesn't melt below 160oC, or 320oF. Polyethylene, a more common plastic, will anneal at around 100oC, which means that polyethylene dishes will warp in the dishwasher. As a fiber, polypropylene is used to make indoor-outdoor carpeting, the kind that you always find around swimming pools and miniature golf courses. Structurally, it's a vinyl polymer, and is similar to polyethylene, only that on every other carbon atom in the backbone chain has a methyl group attached to it.
Methyl methacrylate. Production and properties[edit] The compound is manufactured by several methods, the principal one being the acetone cyanohydrin (ACH) route. PMMA.