

A Female Surgical Nightmare. How a problematic medical device has escaped FDA regulation.
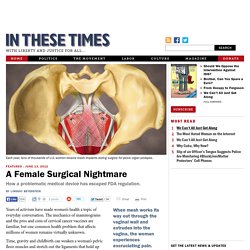
When mesh works its way out through the vaginal wall and extrudes into the vagina, the woman experiences excruciating pain. During intercourse, mesh can lacerate her partner's penis. Years of activism have made women’s health a topic of everyday conversation. The mechanics of mammograms and the pros and cons of cervical cancer vaccines are familiar, but one common health problem that affects millions of women remains virtually unknown. Time, gravity and childbirth can weaken a woman’s pelvic floor muscles and stretch out the ligaments that hold up her uterus, bladder and/or bowel, causing the organs to sag and bulge into the vagina—or even protrude from it—a disorder known as pelvic organ prolapse (POP).
The symptoms depend on which organs are bulging and how much. The condition can be debilitating and demoralizing—though rarely life-threatening. Top 10 Things to Know About Mesh BEFORE Having Surgery for POP or SUI. What if I could go back in time to before I had my mesh implant for SUI?
Suspension of mesh surgery in Scotland means women need improved access to physiotherapy. Mesh Victims Need Help NOW: Go Fund Me. March 15, 2015 Many stories have been written on this site about transvaginal mesh and the complications and many brave victims have shared their stories about being sold on this “simple little procedure” that was supposed to make their lives better not worse and how its negatively impacted not only their lives but the lives of their family members as well.
To many who read this site they live these problems day in and day out. To many who read this site this is just someone else’s problem and as a society (myself included) we tend to not pay attention to things unless they directly involve us. That said, one story I recount outside the mesh community engages the otherwise unaware and garners a Pavlovian response in males: “Can you imagine having sex and getting your penis shredded because your wife’s mesh perforated the vaginal wall?” Medical Council stops Reid from doing major surgery. THE NSW Medical Council placed Dr Richard Reid under practice conditions in 2011, including that he obtain ‘‘valid informed consent for the surgery he performs’’, after complaints and at least three civil settlements against him.
Dr Richard Reid was barred by the Medical Council from performing major surgery in March this year, while the Tissue Fixation System was deregistered last November. Botched surgery claims led to $7.6m payout. Links to Medical Mesh Research - Links to Medical Mesh Research. Summary_of_the_evidence_on_the_benefits_and_risks_of_vaginal_mesh_implants. First Do No Harm: Complications and Complaints will Change Pelvic Floor Surgery Using Mesh Implants in 2015. IntroductionIn a plenary session of the Scottish parliament on the 26th of June 2014 Alex Neil the Scottish cabinet minister for social justice gave a ministerial statement on the use of polypropylene (plastic) mesh devices in pelvic floor surgery.
Engeland - United Kingdom.... Scottish Meshsurvivors. Franstalige artikels- articles Francais. Medische Artikels- Medical Articles- drs. opinions.. Media - program - news- radio. Over implantaten - about medical devices (implants) Fonds MO-Rechtszaken-legal news... Matjes- mesh complications. Europees - Wereldwijd onderzoek naar matjes. Bekkenbodem- prolaps / therapie / Brochure. Bekkenbodem-patiënten verenigingen. NVOG -Ned. gynaecologen vereniging- IGZ. Www.Meshedup.eu - testaankoop - BEUC - Europa.
Tros Radar-bekkenbodemmatjes. Pacemakers are not vacuum cleaners. Towards new guidelines for the introduction of novel medical devices in pelvic floor surgery - Facts, Views & Vison in obGyn. Our Belgian lay press recently became aware of the trend amongst medical professionals that the introduction of novel medical devices may need some more formal over- sight.
For making that point, they were quoting from a de- bate during a cardiologist’s meeting, with a one liner that the “requirements for bringing a new pacemaker or stent onto the market is from a regulatory viewpoint as simple as marketing a new vacuum cleaner” (Een pacemaker is geen stofzuiger. De Standaard, 1/9/2011, Figure 1). Patients would get scared for less ! Apparently, the press missed July 13th, 2011 the up- dated health notification from the Food and Drug Administration (FDA) on the surgical placement of mesh via the vagina to repair pelvic organ prolapse (POP) (es- sentials summarized in Table 1) ( Med- icalDevices/Safety/AlertsandNotices/ucm262435.htm#.Th3ej3Bki9s.email).
It bluntly states that vaginal mesh placement “may expose patients to greater risk than other surgical options”. J. Dr. Mueller Uses Ultrasound to Identify Surgical Mesh. MRI will not show the placement of mesh and neither can an X-Ray.

However, one modality that can depict mesh is ultrasound. According to Dr. Elizabeth Mueller, Division and Fellowship Director of Female Public Medicine and Reconstructive Surgery at Loyola University Chicago Stritch School of Medicine, ultrasound is the only modality that allows her to not only detect mesh, but perform more precise mesh removal surgeries. We had the privilege of interviewing Dr. OK van de toekomst - Antoni van Leeuwenhoek. Comment made by meshsister. FDA: vaginal mesh reclassified /stronger regulation /class III. Boston Scientific accused of using counterfeit ingredients - Crossroads Today.
By Jackie Wattles NEW YORK (CNNMoney) -- A class action lawsuit against Boston Scientific made public Thursday alleges the company used counterfeit material from China to manufacture toxic vaginal mesh implants.
The suit filed in a West Virgina federal court is yet another in the mounting litigation that's been brought against makers of vaginal mesh implants. Patients have said the devices caused miscarriages and other severe medical issues. A few cases have been settled, and more than 70,000 civil lawsuits have been consolidated into an ongoing case playing out in federal court. The West Virgina suit goes beyond the claims made in those cases.
If the allegations are substantiated, Boston Scientific could face criminal charges and even heftier payouts than it would in other civil cases. At issue is an ingredient called resin. Amber Mostyn, the lead attorney in the case, said the primary goal is to get an injunction to halt the sale of Boston Scientific's vaginal mesh implants. Victims of Transvaginal Mesh Devices Finally Get the Justice they Deserve. Two victims of what has been called “one of the biggest U.S. mass torts in history” will finally get some justice, thanks to a ruling issued yesterday by the U.S.
Court of Appeals for the Fourth Circuit in Cisson v. C.R. Bard, Inc. Over 70,000 more cases are waiting in the wings. The appeal involves transvaginal mesh medical devices, used to treat pelvic organ prolapse in women. In yesterday’s decision, the Fourth Circuit affirmed the first jury verdict arising from multi-district litigation involving more than 70,000 cases against the proprietors of transvaginal mesh medical devices, which awarded the plaintiffs—Donna Cisson and her husband Don—$250,000 in compensatory damages and $1,750,000 in punitive damages against device manufacturer C.R.
The decision is important news for the Cissons. Mostyn Law: RICO case targets company over tainted vaginal implants. HOUSTON, Jan. 14, 2016 /PRNewswire-USNewswire/ -- Mostyn Law, a Houston-based firm, has accused a leading U.S. medical company of running an international conspiracy that sold defective vaginal surgical mesh, made of counterfeit supplies it smuggled from China.
The firm, headed by attorneys Amber and Steve Mostyn, sued Boston Scientific Corp. and three other companies under the Racketeering and Corrupt Organizations Act (RICO) on behalf of women who have suffered severe discomfort, bleeding, infections, painful intercourse, urinary problems and other complications from the plastic mesh implants. The class-action lawsuit says that after losing its U.S. supplier of the synthetic resin to produce the mesh, Boston Scientific bought unverified, substandard material from a known counterfeiter in China. The company took extraordinary measures to avoid being caught by U.S. and Chinese authorities, at times acting like a drug dealer to hide multiple overseas shipments, the suit says. Mostynemail.docx. Boston Sci Accused of Racketeering, Using Counterfeit Resin.
Boston Scientific has been sued under the Racketeering and Corrupt Organizations Act (RICO) for allegedly orchestrating a conspiracy to sell misbranded transvaginal mesh made from counterfeit plastic smuggled from China. Qmed News A federal recent lawsuit filed in West Virginia observes that, despite the controversy surrounding transvaginal mesh, Boston Scientific still collects $120 million in revenue on average from the sale of the product, which is implanted in roughly 55,000 women each year.