

The 2012 Enigma by David Wilcock Pt. 02. Time Travel. Time travel. Quantum mechanics. Wavefunctions of the electron in a hydrogen atom at different energy levels.
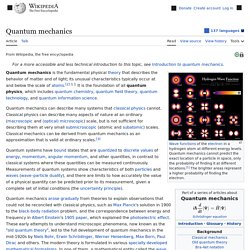
Quantum mechanics cannot predict the exact location of a particle in space, only the probability of finding it at different locations.[1] The brighter areas represent a higher probability of finding the electron. Quantum mechanics (QM; also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of atoms and subatomic particles.[2] Quantum mechanics gradually arose from theories to explain observations which could not be reconciled with classical physics, such as Max Planck's solution in 1900 to the black-body radiation problem, and from the correspondence between energy and frequency in Albert Einstein's 1905 paper which explained the photoelectric effect.
History[edit] In 1838, Michael Faraday discovered cathode rays. Confluent hypergeometric function. In mathematics, a confluent hypergeometric function is a solution of a confluent hypergeometric equation, which is a degenerate form of a hypergeometric differential equation where two of the three regular singularities merge into an irregular singularity.
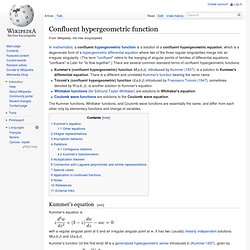
(The term "confluent" refers to the merging of singular points of families of differential equations; "confluere" is Latin for "to flow together".) There are several common standard forms of confluent hypergeometric functions: Kummer's (confluent hypergeometric) function M(a,b,z), introduced by Kummer (1837), is a solution to Kummer's differential equation. The Kummer functions, Whittaker functions, and Coulomb wave functions are essentially the same, and differ from each other only by elementary functions and change of variables.