

31.03.21 WHO 2019 nCoV therapeutics. WHO COVID-19 Clinical management: living guidance. Overview.

23.03.21 NICE covid19 rapid guideline managing covid19 children and adults. NHScovid19treatmentguidelines. 2020 06 20 BOE Medicamentos esenciales COVID. BMJ Best Practices Management of coexisting conditions in the context of COVID 19 12. 10.04 recommendations checklist and tables Universidad de Maryland. 10.04 Guía optimización terapéutica COVID long term facilities Universidad de Maryland.
Sociedades Científicas. Coronavirus - ASHP. Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first reported in Wuhan, China, that is now spreading in the United States and globally.
ASHP’s COVID-19 Resource Center aims to provide evidence-based information and tools for pharmacists, who play a key role as patient care providers and in implementing infection control procedures, and other healthcare professionals. ASHP Resources Tools and Guidance News and Podcasts Policy & Advocacy Connect Community. Canadá Treatment Archives - This interim guidance was developed for clinicians caring for patients with confirmed infection with SARS-CoV-2, the virus that causes COVID-19.

The CDC will update this interim guidance as more information becomes available. Link to Report Clinical Care Guidance The WHO has provided a practical manual for setting up and managing a severe acute respiratory infection treatment centre and screening facility in health care facilities. The document has been developed to meet the operational needs emerging with the COVID-19 pandemic. Link to Report Severe Acute Respiratory Infections Treatment Centre The Canadian Pharmacists Association (CPhA) has published the following guidance for pharmacists regarding disease-modifying antirheumatic drugs (DMARDs): “To date, there is no data on COVID-19 in patients with rheumatic disease taking DMARDs.
BMJ 02-03-21 A living WHO guideline on drugs to prevent covid-19. This is a living guideline, so the recommendations included here will be updated, and new recommendations will be added for other prophylactic drugs for covid-19.
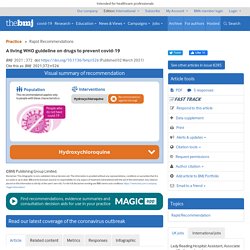
The infographic provides a summary of the recommendations and includes links to the MAGICapp for more details on the evidence and rationale for the recommendation, as well as patient decision aids. Box 2 outlines key methodological aspects of the guideline process. Box 2. BMJ A living WHO guideline on drugs for covid-19. This is a living guideline, so the recommendations included here will be updated, and new recommendations will be added on other therapies for covid-19.
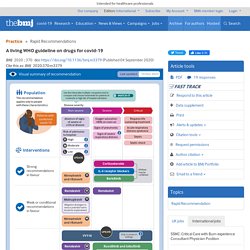
The infographic provides a summary of the recommendations and includes links to the MAGICapp for more details on the evidence and rationale for the recommendation, as well as patient decision aids. Box 2 outlines key methodological aspects of the guideline process. Box 2. Bmj.10.03.21 Neumonía por Covid severa dg y manejo.
Biológicos y citokinas. Plasma de curados. Corticoides. HBPM y ACO. Remdesivir. Azitromicina. Aspirina. Povidona enjuagues. MPR 16.12 Metformin Use May Reduce Mortality in Women With COVID 19. MPR 29.01.21 Metformin Use for T2DM May Reduce COVID 19 Mortality. Ciclosporina, el prometedor fármaco que reduce un 81% las probabilidades de fallecer por Covid-19. Actualizado:16/10/2020 10:29h Guardar Los pacientes de Covid-19 que fueron tratados con ciclosporina en el Hospital Universitario Quirónsalud Madrid tuvieron un 81 por ciento menos de probabilidad de fallecer que aquellos enfermos que no recibieron este fármaco.
Esta es la principal conclusión a la que ha llegado el Grupo de Investigación en Covid-19, liderado por el Servicio de Medicina Interna de dicho hospital, junto con la Universidad Europea, tras realizar un estudio retrospectivo que analiza los tratamientos de más de 600 pacientes con coronavirus ingresados en este centro sanitario desde el 10 de marzo hasta el 15 de abril de 2020. Los resultados se publican la revista «E Clinical Medicine», del grupo «The Lancet». «Los pacientes incluidos en este estudio sufrían afectación respiratoria suficientemente importante para necesitar ingreso hospitalario.
Hemos analizado sus características clínicas y hemos analizado tratamientos que recibieron. Ver los comentarios. Nejm 02.12 Solidiraty interim results. Nejmoa 02.12 Reposiciconar antivirales Ed. MPR 26.01.21 WHO Remdesivir, Hydroxychloroquine, Lopinavir, Interferon Ineffective for COVID 19. Guía NICE Vitamina D y paso a UCI 28. Diabetes care 29.09 Sitagliptina y mortalidad COVID. MPR 03.02.21 Umifenovir Contributes to Clinical and Laboratory Improvement of COVID 19 Management. NEJM Lopi Rito. MPR 13.10 Lopi Rito no eficaces. MEDJ 17.04 L R vs Albidol. MPR 24.04 L R vs Albidol. Lancet 08.05 Triple tp L R Interferón Ribavirina. Lancet Resp Med nov interferon inhalado.
Can Cholesterol Drugs and Antihistamines Fight COVID-19? AAEM 06.04 RS Tto antiviral. MPR 15.03.21 Study Findings Do Not Support Use of Ivermectin for Mild COVID 19. FDA 03.05.21 Why You Should Not Use Ivermectin to Treat or Prevent COVID-19. Image COVID-19.
We’ve been living with it for what sometimes seems like forever. Given the number of deaths that have occurred from the disease, it’s perhaps not surprising that some consumers are looking at unconventional treatments, not approved or authorized by the Food and Drug Administration (FDA). Though this is understandable, please beware. The FDA’s job is to carefully evaluate the scientific data on a drug to be sure that it is both safe and effective for a particular use, and then to decide whether or not to approve it. Medscape 08.03.21 FDA Covid Ivermectina. Jama lpezmedina 04.03. 2021 EC Ivermectina. DICAF Ivermectina in vitro COVID19. MPR 21.05 IVIG en COVID grave. MPR 21.05 IVIG. 28.08 Estatinas y COVID. MPR 29.06 Estatinas y COVID19. MPR 29.06 Estatinas y COVID. MPR 23.04 Dapagliflozina COVID.
Medscape 30.04 Dapagliflozina para COVID. MPR 26.01.21 WHO Remdesivir, Hydroxychloroquine, Lopinavir, Interferon Ineffective for COVID 19. MPR 10.06 Flora intestinal para COVID. Gut Famotidina y COVID. MPR 11.06.Case Series Suggests Famotidine May Improve COVID 19 Symptoms. Famotidine: New York hospitals studying heartburn drug as Covid-19 treatment. Preliminary results of the clinical trial of famotidine, the active ingredient in Pepcid, could come out in the next few weeks, said Dr.
Kevin Tracey, president of Feinstein Institutes for Medical Research at Northwell Health, which runs 23 hospitals in the New York City area. So far, 187 patients have enrolled in the clinical trial, and Northwell eventually hopes to enroll 1,200, he said. "There are many examples in the history of medicine where a drug that was designed for one purpose turns out to have an effect in another disease," Tracey said. He said if famotidine works -- and that's a big if at this point -- it would be easy to use it on a widespread scale. "It's generic, it's plentiful and it's inexpensive," he said. But he emphasized that it might not work.
"We don't know if it has any benefit. He also emphasized that the patients in the study are in the hospital taking mega-doses intravenously -- doses about nine times what someone would normally take for heartburn. 28.10 MPR COVID 19 Famotidine Associated With Improved Outcomes in Hospitalized Patients. Science Magazine 26.04 Famotidina EC. Science’s COVID-19 reporting is supported by the Pulitzer Center.
The fast-growing list of possible treatments for the novel coronavirus includes an unlikely candidate: famotidine, the active compound in the over-the-counter heartburn drug Pepcid. On 7 April, the first COVID-19 patients at Northwell Health in the New York City area began to receive famotidine intravenously, at nine times the heartburn dose. Unlike other drugs the 23-hospital system is testing, including Regeneron’s sarilumab and Gilead Sciences’s remdesivir, Northwell kept the famotidine study under wraps to secure a research stockpile before other hospitals, or even the federal government, started to buy it. “If we talked about this to the wrong people or too soon, the drug supply would be gone,” says Kevin Tracey, a former neurosurgeon in charge of the hospital system’s research. Related A globe-trotting infectious disease doctor named Michael Callahan was the first to call attention to the drug in the United States. Medscape 05.03.21 Recovery para el brazo de colchicina.
Medscape 09.02.21 Colchicina y Covid. 14.02.21 Quid pro quo: La colchicina y la infección por la COVID-19. BMJ SEPT 20 Drug treatments for covid-19: living systematic review and network meta-analysis. Abstract Objective To compare the effects of treatments for coronavirus disease 2019 (covid-19).
Design Living systematic review and network meta-analysis. Data sources WHO covid-19 database, a comprehensive multilingual source of global covid-19 literature, up to 3 December 2020 and six additional Chinese databases up to 12 November 2020. Study selection Randomised clinical trials in which people with suspected, probable, or confirmed covid-19 were randomised to drug treatment or to standard care or placebo. Pairs of reviewers independently screened potentially eligible articles. BMJ 28.04 RECOVERY Trial UK. RECOVERY protocol. Medscape 14.08 Metformina favorable en COVID. Thomas 12.02.21 Zinc. ascórbico y Covid. Clin Infect Dise Oct COVID y prolongación QT. Liverpool COVID-19 Interactions. Covid InteractionDetails Web 2020 Apr 09. Interacciones de los fármacos anticoagulantes con terapias COVID-19 (From ESC European Society Cardiology) COVID Interacciones ACO.
Ensayos clínicos. Tratamiento COVID y QT. Preevid: banco de preguntas COVID Tratamiento. COVID 19 Summary and References extraido de UW 23. COVID-19: An ACP Physician's Guide + Treatment: Pharmacotherapy. The safety and effectiveness of combination therapy remains under scrutiny. Major guidance statement recommend against use of this drug combination, based on lack of significant evidence of benefit as well as concerns for adverse effects including QTc prolongation.
Initial interest in this combination was sparked by a small, non-peer-reviewed, preprint, open-label, non-randomized, practical study that enrolled 26 patients [6 asymptomatic, 22 with URI symptoms, and 8 with LRTI symptoms] and 16 controls to observe outcomes on viral load at day 6 of treatment31. Of the 26 patients in the treatment group, 6 also received azithromycin with daily electrocardiographic monitoring) and these 6 demonstrated more rapid resolution of viral load in nasopharyngeal swabs31. Aside from the small sample size, other limitations of the study included the method of identifying controls, confounders of baseline viral load, imputed data, and loss of patient follow up data51.
Thorax 2020 EPOC y COVID. NIH Guidelines COVID-19 abril20. 13.04 Revisión tto farmacológico COVID JAMA. CMAJ revisión tto 29. OSU 13.04 manejo COVID. ASHP COVID 19 Evidence Table 01.04. ASHP COVID 19 Evidence Table 24.03. Canadá British Columbia 12.04 Guidelines Unproven Therapies COVID 19. Am Thoracic Soc COVID guidance 03.04. Infectious Disease Society of America 11.04 guideline tratamiento COVID. L. Spallanzani IRCCS. Recommendations for COVID 19 clinical management. Yale University 03.04 COVID 19 algoritmo con resumen de ttos.
NEJM Drug Evaluation COVID. NICE covid19 rapid guideline managing symptoms at the end of life 03.04. COVID-19 rapid guideline: acute myocardial injury. The purpose of this guideline is to help healthcare professionals who are not cardiology specialists identify and treat acute myocardial injury and its cardiac complications in adults with known or suspected COVID-19 but without known pre-existing cardiovascular disease. This guideline is for: health and care practitionershealth and care staff involved in planning and delivering servicescommissioners The guideline covers care in a hospital setting. The recommendations are specifically for acute myocardial injury associated with COVID-19 and should be used alongside good clinical practice. The recommendations bring together: evidence from published literature on COVID-19 with acute myocardial injuryexisting national and international guidance and policiesadvice from specialists working in the NHS from across the UK.
NICE has developed these recommendations in direct response to the rapidly evolving situation and so could not follow the standard process for guidance development. COVID-19 rapid guideline: gastrointestinal and liver conditions treated with drugs affecting the immune response. The purpose of this guideline is to maximise the safety of children and adults who have gastrointestinal or liver conditions treated with drugs affecting the immune response during the COVID 19 pandemic. It also aims to protect staff from infection and enable services to make the best use of NHS resources. NICE has developed these recommendations in direct response to the rapidly evolving situation and so could not follow the standard process for guidance development.
The guideline has been developed using the interim process and methods for developing rapid guidelines on COVID-19. The recommendations are based on evidence and expert opinion and have been verified as far as possible. We will review and update the recommendations as the knowledge base and expert experience develops. 15.05. NICE covid19 rapid guideline chronic kidney disease. Antivirales anticuerpos Public Health Agency of Canada 25.03.
Am J Gastroenterol PPI y riesgo COVID julio.