

Valence (chemistry) Chemical symbol. Boiling point. Boiling water The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor.
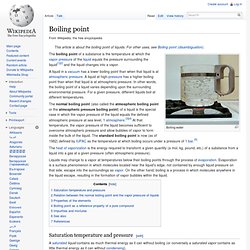
A liquid in a vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at high-pressure has a higher boiling point than when that liquid is at atmospheric pressure. Density. Where ρ is the density, m is the mass, and V is the volume.
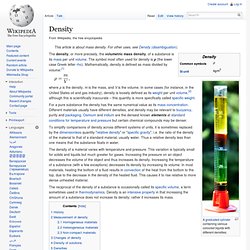
In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume,[2] although this is scientifically inaccurate – this quantity is more specifically called specific weight. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative density" or "specific gravity", i.e. the ratio of the density of the material to that of a standard material, usually water. Thus a relative density less than one means that the substance floats in water. The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Melting point. The melting point (or, rarely, liquefaction point) of a solid is the temperature at which it changes state from solid to liquid at atmospheric pressure.
At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends (usually slightly) on pressure and is usually specified at standard pressure. Atomic weight. Relative atomic mass (symbol: Ar) is a dimensionless physical quantity, the ratio of the average mass of atoms of an element (from a single given sample or source) to 1/12 of the mass of an atom of carbon-12 (known as the unified atomic mass unit).[1][2] The term is equivalent to atomic weight, which is the older term, and which is also sample (source) specific.
Thus, two samples of a chemical element which is naturally found as being composed of more than one isotope, collected from two substantially different sources, are expected to have slightly different relative atomic masses (atomic weights), because isotopic concentrations typically vary slightly with the history of the source. Both the terms relative atomic mass and atomic weight are sometimes loosely used to refer to a technically different standardized expectation value, called the standard atomic weight. Atomic number. An explanation of the superscripts and subscripts seen in atomic number notation.
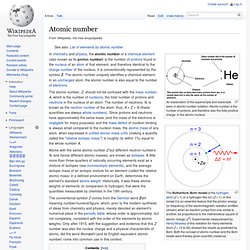
Atomic number is the number of protons, and therefore also the total positive charge, in the atomic nucleus. The Rutherford–Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1). In this model it is an essential feature that the photon energy (or frequency) of the electromagnetic radiation emitted (shown) when an electron jumps from one orbital to another, be proportional to the mathematical square of atomic charge (Z2).
Experimental measurement by Henry Moseley of this radiation for many elements (from Z = 13 to 92) showed the results as predicted by Bohr. Both the concept of atomic number and the Bohr model were thereby given scientific credence. Halogen. History[edit] The fluorine mineral fluorospar was known as early as 1529.
Early chemists realized that fluorine compounds contain an undiscovered element, but were unable to isolate it. In 1869, George Gore, an English chemist, ran a current of electricity through hydrofluoric acid and discovered fluorine, but he was unable to prove his results at the time. In 1886, Henri Moissan, a chemist in Paris, performed electrolysis on potassium bifluoride dissolved in waterless hydrofluoric acid, and successfully produced fluorine.[1] Hydrochloric acid was known to alchemists and early chemists. Bromine was discovered in the 1820s by Antoine-Jérôme Balard. Iodine was discovered by Bernard Courtois, who was using seaweed ash as part of a process for saltpeter manufacture. In 1931, Fred Allison claimed to have discovered element 85 with a magneto-optical machine, and named the element Alabamine, but was mistaken. Etymology[edit] Fluorine's name comes from the Latin word fluere, meaning "to flow". Noble gas. The noble gases make a group of chemical elements with similar properties: under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity.
The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). For the first six periods of the periodic table, the noble gases are exactly the members of group 18 of the periodic table. It is possible that due to relativistic effects, the group 14 element flerovium exhibits some noble-gas-like properties,[1] instead of the group 18 element ununoctium.[2] Noble gases are typically highly unreactive except when under particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted.
For example: argon is used in lightbulbs to prevent the hot tungsten filament from oxidizing; also, helium is breathed by deep-sea divers to prevent oxygen and nitrogen toxicity. Biochemistry. Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life.
Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology. Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism.
History[edit] Lipids[edit] Molecular biology. Mole (unit) Periodic table. Standard 18-column form of the periodic table. Alchemy. The Emerald Tablet, a key text of Western Alchemy, in a 17th-century edition Alchemy is an influential philosophical tradition whose practitioners have, from antiquity, claimed it to be the precursor to profound powers.
Carbonated water. A glass of sparkling water Carbonated water (also known as club soda, soda water, sparkling water, seltzer water, or fizzy water) is water into which carbon dioxide gas under pressure has been dissolved.
This process, known as carbonation, is a process that causes the water to become effervescent. Carbonated water is the defining ingredient of carbonated soft drinks. Etymology[edit] Carbonic acid. Not to be confused with carbolic acid, an antiquated name for phenol. Carbonic acid is also an archaic name for carbon dioxide. Chemical equilibrium[edit] When carbon dioxide dissolves in water it exists in chemical equilibrium producing carbonic acid:[2] The hydration equilibrium constant at 25°C is called Kh, which in the case of carbonic acid is [H2CO3]/[CO2] ≈ 1.7×10−3 in pure water[3] and ≈ 1.2×10−3 in seawater.[4] Hence, the majority of the carbon dioxide is not converted into carbonic acid, remaining as CO2 molecules.
Ocean acidification. NOAA provides evidence for upwelling of corrosive "acidified" water onto the Continental Shelf. In the figure above, note the vertical sections of (A) temperature, (B) aragonite saturation, (C) pH, (D) DIC, and (E) pCO2 on transect line 5 off Pt. St. George, California.