

Finally, the drug that keeps you young. The Top 8 Things to Know About Anti-Aging Research Right Now - leapsmag. Kira Peikoff Editor-in-Chief show profile Dr.
Michael West has a storied legacy in the world of aging research. Twenty years ago, the company he started, Geron, hit upon a major breakthrough when his scientists isolated the active component for the gene that confers immortality to cells, called telomerase. In the twenty years since, a new field has emerged: the science of extending the human “healthspan.
" À propos de nous - Long Long Life - Work for human longevity. Nos fondateurs Le travail de recherche a un coût.
Partie 1 : Parlons peu, parlons génomique ! - Work for human longevity. Références [1] R.W.
Holley, et al., Structure of a ribonucleic acid, Science, 1965;147:1462–1465 [2] Sanger, et al., Nucleotide sequence of bacteriophage phi X174 DNA, Nature 1977;265:687–695 [3] F. This Scientist Predicted He Would Live to 150. Now He's Not So Sure. Alex Zhavoronkov was still young when he became obsessed with pushing the boundaries of the human lifespan.
In his almost 20-year career, he’s worked as a computer scientist and biotechnology researcher. Now 38, Zhavoronkov is working with artificial intelligence and big data at Insilico, a biotech startup where he’s the CEO. And he firmly believes that aging research is the most important field of science right now. In the past, Zhavoronkov claimed that he expected to live to be 150, but now he’s more conservative. Les causes biologiques du vieillissement et de la limitation de la durée de vie - Work for human longevity. En 2014, des scientifiques ont démontré que le sang jeune pouvait rajeunir de vieux tissus en combinant ces deux éléments chez les souris [130].
De plus, les manipulations qui prolongent la durée de vie ciblent un tissu qui pourrait avoir des conséquences bénéfiques sur d’autres [131] [132] [133]. How to Cure Aging – During Your Lifetime? Naked mole rats defy the biological law of aging. In the world of animal models, naked mole rats are the supermodels.
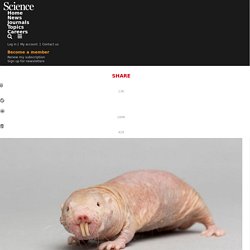
They rarely get cancer, are resistant to some types of pain, and can survive up to 18 minutes without oxygen. But perhaps their greatest feat, a new paper suggests, is that they don't age. The first study to analyze the life histories of thousands of naked mole rats has found that their risk of death doesn't go up as they grow older, as it does for every other known mammalian species. Although some scientists caution against any sweeping conclusions, many say the new data are important and striking. Could Filtering Our Aged Blood Keep us Young? Nos Objectifs. Stimuler, promouvoir et informer les citoyens à propos de l’allongement de la vie atteinte par toutes les sortes de technologies biogérontologiques entre autres SENS, la restriction calorique, la recherche génétique, les procédés de régénération,… Activités Ceci peut être atteint par l’organisation d’exposés tant pour le grand public que pour des scientifiques, des sessions d’information, des rencontres ainsi que la diffusion d’informations relatives à l’allongement de l’espérance de vie par des journaux, internet (sites, courriels, lettres d’information,…), des périodiques, des dépliants, des posters et toutes autres activités qui peuvent aider à atteindre l’objectif en Belgique ou dans d’autres pays.
Young Again: How One Cell Turns Back Time. Silicon Valley : la course contre la mort a commencé. How Scientists Will Beat Aging in Our Lifetimes. Even in 2017, anti-aging and lifespan-increasing technology still feels futuristic.

But this new video from Kurzgesagt presents several technologies close to completion that could make a big impact on how we age. Advertisement - Continue Reading Below The first of these is a way to kill off senescent cells--zombie cells that clog up your body and disrupt normal functioning. These dead cells hang around and cause problems, and up till now, there's been no way to get rid of them. Genes for Healthy Aging Found. In addition to the genes associated with longevity, say Chinese scientists, there are genes associated with age-related behavioral decline.

The scientists decided to substantiate this assertion by studying differences in the rate of aging in wild strains of Caenorhabditis elegans, while also measuring the rates of age-related decline in traits associated with mating, feeding, and locomotion. Pourquoi vieillissons-nous? Chacun sait que notre organisme est appelé à décliner avec l’âge. En s’efforçant de comprendre pourquoi, les chercheurs espèrent pouvoir un jour ralentir voire enrayer ce processus. Lutter contre le vieillissement en s'injectant de l'ADN modifié ? - Sciencesetavenir.fr. Se transformer en OGM avec l'objectif de rajeunir, c'est la déroutante expérience menée depuis 2015 par l'américain Brian Hanley, fondateur de l'entreprise de microbiologie Butterfly Sciences.
Mitochondria/cell damage. Protein injection. Telomere length increasing. Avoid mitophagy. Young blood transfusion. Susan Pinker: The secret to living longer may be your social life. Letemps. Compared to Other Organisms, Humans Really Don't Live Very Long. Humans are lucky if they ever make it to 100 years old; the average person lives just 3/4 of that figure. On the other hand, other organisms can easily out-live human beings. Silicon Valley : la course contre la mort a commencé. ?articles. ISTOCK, DRA_SCHWARTZIndividuals who possess an innate resilience to age-related brain pathologies may offer molecular clues to unexplored therapeutics for neurodegenerative disease.
After having accidentally discovered rapid aging and disease in mice with mutations in the gene that encodes the protein klotho—named after the Greek Fate Clotho, daughter of Zeus and spinner of the thread of life—independent researchers have shown that some people with genetic variants that promote elevated klotho levels live longer and tend to stave off age-related cognitive decline. Notably, in each type of mouse, the protein fragment was injected into the animals’ bodies either a day or a few hours before cognitive testing took place.
Previously, neurologist and researcher Dena Dubal of the University of California, San Francisco, and others have demonstrated that transgenic overexpression of klotho throughout an organism’s lifespan produces similar cognitive improvements. ?articles. A deletion in a growth hormone receptor gene is tied to an average of 10 extra years of life among men, but not women, according to a study. FLICKR, GABRIEL ROCHANailing down genetic factors linked to longevity in humans has proven challenging, but a new study points to a deletion involving growth hormone receptor gene’s exon 3 (d3-GHR) as possibly playing a significant role. Among 841 people from long-lived populations, the proportion of individuals carrying two copies of d3-GHR increased with age. The effect was specific to men, who lived some 10 years longer than those without the mutation. The results were published Friday (June 16) in Science Advances.
With the d3-GHR deletion, “you still have a functional protein that now makes people live longer. “The results look convincing to me,” Ali Torkamani, the director of genome informatics at the Scripps Translational Science Institute in La Jolla, California, who was not involved in the study, told The New York Times. Science News, Webinars and Virtual Events.
We all accept that aging is a natural part of growing up. The human body is not built to last forever. Long Long Life : mécanismes biologiques du vieillissement. L’âge et la vieillesse, qui vont aujourd’hui main dans la main, sont souvent associés à un large faisceau de maladies dites « liées au vieillissement » : maladies neurodégénératives, problèmes cardiaques… Afin d’apprendre comment les prendre en charge au mieux, la recherche s’intéresse à ce qui entraîne leur apparition, à savoir, la vieillesse elle-même. Major research initiative explores how our bones and muscles age, new ways to block their decline. AUGUSTA, Ga. (June 6, 2017) – With age, the form and function of our bones and muscles drop off, putting us as increased risk for frailty and falls. Now researchers at the Medical College of Georgia at Augusta University are dissecting just what happens to the stem cells that make the tissues, which help keep us upright, with an eye on improving our healthspan.
Osteoporosis already is a major public health problem affecting about 44 million Americans and costing billions annually. The world's older population is growing at an unprecedented rate with 8.5 percent of the worldwide population – 617 million people – age 65 and older, a proportion estimated to reach 17 percent by 2050, according to the National Institute on Aging. "After age 65 you start losing about 1 percent of both muscle and bone per year," said Dr. Dan Buettner: How to live to be 100+ High levels of exercise linked to nine years of less aging at the cellular level. Despite their best efforts, no scientist has ever come close to stopping humans from aging. Even anti-aging creams can't stop Old Father Time. But new research from Brigham Young University reveals you may be able to slow one type of aging—the kind that happens inside your cells.
As long as you're willing to sweat. "Just because you're 40, doesn't mean you're 40 years old biologically," Tucker said. ?articles. © BRYAN SATALINOInjecting a protein derived from human umbilical cord plasma—tissue inhibitor of metalloproteinases 2 (TIMP2)—into aged mice led to improvements in the rodents’ learning, memory, and synaptic plasticity, researchers reported today (April 19) in Nature. “Following our previous observations that young mouse plasma can functionally improve the behavior [of old mice], this study now shows that human blood may actually have similar factors,” coauthor Tony Wyss-Coray of the Stanford University School of Medicine in Palo Alto, California, told The Scientist.
The authors “went the extra mile to demonstrate that this protein, TIMP2, is in fact enriched in umbilical cord plasma, but also that it’s a systemic factor that is found in young mice and then decreases in mice with aging,” said Greg Valdez of the Virginia Tech Carilion Research Institute in Roanoke, who did not participate in the work. “It seems to have pro-plasticity effects in these aged mice, which is terrific. J.M. Craig Venter Mapped The Genome. Now He's Trying To Decode Death. Serait-il bientôt envisageable d'arrêter de vieillir? Une découverte réalisée par une équipe internationale de chercheurs pourrait conduire à la mise au point d'un médicament pour le moins révolutionnaire, qui arrêterait le processus du vieillissement, améliorerait la réparation de l'ADN et pourrait même aider des astronautes à se rendre sur Mars. Debilities of Aging Reversed by Protein That Targets Senescent Cells. Relieved of the burden of senescent cells, we may enjoy extended healthspans—or even lifespans.
This notion was put the to the test by a team of scientists based at Erasmus University Medical Center. They selectively eliminated senescent cells in aging mice, which responded by showing a reversal of age-related loss of fur, poor kidney function, and frailty. When cells become damaged, they resort to senescence—cell cycle arrest—to avoid contributing to the proliferation of flawed cells, which can give rise to diseases such as cancer. Yet senescent cells remain metabolically active, and they secrete factors that promote inflammation.
Rat-taupe nu. Un article de Wikipédia, l'encyclopédie libre. ?articles. WIKIMEDIAIn the nematode worm Caenorhabditis elegans, aging is associated with dysregulation of RNA splicing, according to a paper published on Monday (December 5) in Nature. ?articles. About. Researchers Have Found a "Reset Button" for Aging Cells. Turritopsis nutricula. Will You Age Gracefully? Biomarker Patterns Will Tell. Cerveau : la greffe de neurones n'est plus un rêve. Et si les humains arrêtaient de mourir, de temps en temps ? - Après-demain #005. Jane McGonigal: The game that can give you 10 extra years of life. Will You Age Gracefully? Biomarker Patterns Will Tell. Cellular Reprogramming Rejuvenates Old Mice and Boosts Lifespans 30% Cellular Reprogramming Slows Aging in Mice. Aging and Death Are the Evolutionary Price of Complexity. One Man’s Quest to Hack His Own Genes.
Aging May be Reversible in Mice Trending. ?articles. Longer Life in a Pill May Already Be Available at Your Local Drug Store. Turning Back the Aging Clock. Pourquoi vieillissons-nous? ?articles. The Ageing Process, as Played Out by Our Cells Video. Why the Human Lifespan Ends at 122. Study results advance ‘transposon theory of aging’ Plant Extracts Found to Slow Down Aging Process Trending. ?articles. Virtual Events, Webinars and Videos. Biotech Company Granted Ethical Permission To Attempt To Use Stem Cells To Reactivate The Brains Of The Dead. Ce dérivé de la vitamine B3 peut-il faire rajeunir nos cellules ? Vitamin Found To Halt Aging In Muscle Tissues And Increase Lifespan. Genes identified that extend life. Clearing the Body's Retired Cells Slows Aging and Extends Life. How Old Are You, Really? Biological Age Is Harder to Pin Down Than You Think.
New Technique Extends Lifespan Of Mice By Up To 35 Percent. CHRIS : 5 Trucs à Savoir Sur L'Immortalité. Sans titre. Can Lorenz Studer Cure Parkinson's Disease? Social networks as important as exercise, diet across the span of our lives: Researchers show how social relationships reduce health risk in each stage of life. Worm research in life extension leads scientists to discover new metric to track aging. Longer Life in a Pill May Already Be Available at Your Local Drug Store. Epigenetics of Regeneration. Existing Drug Rejuvenates Brains Of Elderly Rats. Aubrey de Grey: A roadmap to end aging. How Aged Neurons In a Dish Can Accelerate Longevity Research.
Ages apart. Integration of ‘omics’ data in aging research: from biomarkers to systems biology - Zierer - 2015 - Aging Cell. New Test Determines How Fast You're Aging. Steadier Gene Networks Mean Longer Lifespans. Scientists Remotely Activate Genetic Target To Slow Aging Process. Genetic Secrets Of Longevity Discovered. Researchers Discover Off Switch for Ageing Cells. Researchers Discover Unique Aging "Signature" In Brains Of Mice And Humans. Common Painkiller, Ibuprofen, Extends Lifespan Of Several Organisms.
Early Results of Human Trials For Anti-Aging Drug Are Promising. Protein Treatment Staves Off Alzheimer’s Disease Symptoms. How Stem Cells May Save Your Life—and Even Extend It - Singularity HUB. TGen finds gene that gives children appearance of aging - Can aging be treated with drugs? Protein Keeps Aging Heart Young at Heart. What Is The Secret To Eternal Youth? Scientists Reverse Aging In Human Cell Line. Protein-Hoarding Cells Live Longer with Organizer’s Help. XMED: Can We Really Live to 1,000? CNBC Interviews Aubrey de Grey to Find Out.