

Fermi's golden rule. We consider the system to begin in an eigenstate, .
We consider the effect of a (possibly time-dependent) perturbing Hamiltonian, . If. QuantumLevitation. Pauli exclusion principle. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles.
This means that the wave function changes its sign if the space and spin co-ordinates of any two particles are interchanged. Integer spin particles, bosons, are not subject to the Pauli exclusion principle: any number of identical bosons can occupy the same quantum state, as with, for instance, photons produced by a laser and Bose–Einstein condensate. Overview[edit] "Half-integer spin" means that the intrinsic angular momentum value of fermions is (reduced Planck's constant) times a half-integer (1/2, 3/2, 5/2, etc.). Matter wave. The de Broglie relations redirect here.
In quantum mechanics, the concept of matter waves or de Broglie waves /dəˈbrɔɪ/ reflects the wave–particle duality of matter. Holographic principle. In a larger sense, the theory suggests that the entire universe can be seen as a two-dimensional information structure "painted" on the cosmological horizon[clarification needed], such that the three dimensions we observe are an effective description only at macroscopic scales and at low energies.
Cosmological holography has not been made mathematically precise, partly because the particle horizon has a finite area and grows with time.[4][5] The holographic principle was inspired by black hole thermodynamics, which conjectures that the maximal entropy in any region scales with the radius squared, and not cubed as might be expected. Planck scale. In particle physics and physical cosmology, the Planck scale (named after Max Planck) is an energy scale around 1.22 × 1019 GeV (which corresponds by the mass–energy equivalence to the Planck mass 2.17645 × 10−8 kg) at which quantum effects of gravity become strong.
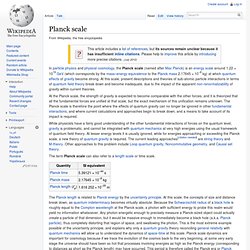
At this scale, present descriptions and theories of sub-atomic particle interactions in terms of quantum field theory break down and become inadequate, due to the impact of the apparent non-renormalizability of gravity within current theories. Uncertainty principle. Where ħ is the reduced Planck constant.
The original heuristic argument that such a limit should exist was given by Heisenberg, after whom it is sometimes named the Heisenberg principle. This ascribes the uncertainty in the measurable quantities to the jolt-like disturbance triggered by the act of observation. Though widely repeated in textbooks, this physical argument is now known to be fundamentally misleading.[4][5] While the act of measurement does lead to uncertainty, the loss of precision is less than that predicted by Heisenberg's argument; the formal mathematical result remains valid, however.
Since the uncertainty principle is such a basic result in quantum mechanics, typical experiments in quantum mechanics routinely observe aspects of it. Certain experiments, however, may deliberately test a particular form of the uncertainty principle as part of their main research program. Introduction[edit] Click to see animation.
Wave mechanics interpretation[edit] . Will we ever overcome the Heisenberg uncertainty principle. Physicist: Nopers!
The Heisenberg uncertainty principle, while normally presented in physics circles, is actually a mathematical absolute. So overcoming the uncertainty principle is exactly as hard as overcoming that whole “1+1=2″ thing. The uncertainty principle (the “position/momentum uncertainty principle”) is generally presented like this: you have some particle floating along and you’d like to know its position and its momentum (velocity, whatever) so you bounce a photon off of it (“Bounce a photon off of it” is just another way of saying “try to see it”).
A general rule of thumb for light (waves in general really) is high frequency waves propagate in straight lines, and low frequency waves spread out. That’s why sunlight (high frequency) seems to go in a perfectly straight line, but radio waves can spread out around corners. So, if you want to see where something is with precision you’ll need to use a high frequency photon. Answer gravy: This gravy has some lumps. . . . . , and . Quantum mechanics. Wavefunctions of the electron in a hydrogen atom at different energy levels.
Quantum mechanics cannot predict the exact location of a particle in space, only the probability of finding it at different locations.[1] The brighter areas represent a higher probability of finding the electron. Quantum mechanics (QM; also known as quantum physics, quantum theory, the wave mechanical model, or matrix mechanics), including quantum field theory, is a fundamental theory in physics which describes nature at the smallest scales of atoms and subatomic particles.[2] Quantum mechanics gradually arose from theories to explain observations which could not be reconciled with classical physics, such as Max Planck's solution in 1900 to the black-body radiation problem, and from the correspondence between energy and frequency in Albert Einstein's 1905 paper which explained the photoelectric effect.
History[edit] In 1838, Michael Faraday discovered cathode rays. Where h is Planck's constant. Coulomb potential.