

Chapter 4, Section 2. Ionic Equations In writing chemical equations for reactions in aqueous solution, it is often useful to indicate explicitly whether the dissolved substances are present predominantly as ions or as molecules.
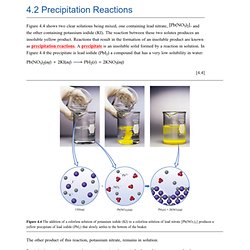
Let's reconsider the precipitation reaction between and 2KI, shown previously in Figure 4.4: KI, and are all soluble ionic compounds and therefore strong electrolytes, we can write the chemical equation to indicate explicitly the ions that are in the solution: Notice that and A net ionic equation includes only the ions and molecules directly involved in the reaction. Charge of the cation and the two charges of the anions add to give zero, the charge of the electrically neutral product. Net ionic equations are widely used to illustrate the similarities between large numbers of reactions involving electrolytes. And any strong electrolyte containing The ions combine to form a precipitate of for example, share many chemical similarities because both contain. Chemical Reactions. Chemical reactions are the processes by which chemicals interact to form new chemicals with different compositions.
Simply stated, a chemical reaction is the process where reactants are transformed into products. How chemicals react is dictated by the chemical properties of the element or compound- the ways in which a compound or element undergoes changes in composition. Describing Reactions Quantitatively Chemical reactions are constantly occurring in the world around us; everything from the rusting of an iron fence to the metabolic pathways of a human cell are all examples of chemical reactions.
Chemistry is an attempt to classify and better understand these reactions. Apologia, ed 1 Chemistry - What will be covered in the Apologia Chemistry Course? Copy of Chemical Reactions Concept Map by katherine Devaroux on Prezi. Shipway.Patrick CMap - What is involved in Chemistry. Apologia, ed 1, Module 11 Concept Map - What is covered in Module 11? Chemical reaction. A chemical reaction is a process that transforms one set of chemical substances to another.
The substances that take part in chemical reactions are known as reactants and the substances produced by the reaction are known as products. The study of chemical reactions is part of the field of science called chemistry.[2] Chemical reactions can result in molecules attaching to each other to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds, and in some types of reaction may involve production of electrically charged end products. Reactions can occur in various environments: solids, liquids, gases, or combinations of same. Chemical reactions can be either spontaneous[4] and require no input of energy, or non-spontaneous[4] which require the input of some form of energy. Zen of Chemistry. Interactive Student Tutorial. Chemistry-batz - 12 Stoichiometry.
"stoy-key-om-a-tree"KEY CONCEPT:Stoichiometry is the study of the mass relationships between substances in a chemical reaction.A balanced chemical equation is the key to solving stoichiometry problems.
Stoichiometry is the quantitative study of chemical reactions. (Stoicheon: element; metron: measure) The coefficients in a balanced chemical equation give the relative amounts of substances involved in a chemical reaction: The most useful way to interpret the coefficients is in terms of moles. Note, only atoms and mass are conserved in a chemical reaction. The following figure shows the calculation for a general "mass-to-mass" stoichiometric conversion: The factors can be adapted to any mass-mass conversion:The mass-moles factor can be found from a periodic table.The balanced equation provides the mole-mole factor. The Stoichiometry "Road Map"The following figure shows the steps for solving a variety of stoichiometry calculations: Class notes with sample calculations. Untuk pelajar.
Precipitation Reactions. c7chemistry.wikispaces. Chapter 3 Chemical Reactions. Chemical Reactivity. Chemical Reactivity Organic chemistry encompasses a very large number of compounds ( many millions ), and our previous discussion and illustrations have focused on their structural characteristics.

Now that we can recognize these actors ( compounds ), we turn to the roles they are inclined to play in the scientific drama staged by the multitude of chemical reactions that define organic chemistry. We begin by defining some basic terms that will be used frequently as this subject is elaborated. Strength of Acids and Bases. What Makes a Strong Acid or Strong Base?
Strong electrolytes are completely dissociated into ions in water. The acid or base molecule does not exist in aqueous solution, only ions. Weak electrolytes are incompletely dissociated. Strong Acids Strong acids completely dissociate in water, forming H+ and an anion. HCl - hydrochloric acid HNO3 - nitric acid H2SO4 - sulfuric acid HBr - hydrobromic acid HI - hydroiodic acid HClO4 - perchloric acid 100% dissociation isn't true as solutions become more concentrated.
Weak Acids A weak acid only partially dissociates in water to give H+ and the anion. Molecules that contain an ionizable proton. Strong Bases Strong bases dissociate 100% into the cation and OH- (hydroxide ion). GCSE Bitesize: Reactions of acids. pH Scale - pH, Hydronium, Concentration. Fundamentals of General, Organic, and Biological Chemistry with MasteringChemistry®, 7/e by John E. McMurry, David S. Ballantine, Carl A. Hoeger & Virginia E. Peterson. Types of Chemical Reactions Ice Cream Form.