

Technology. Home. Common Compound Library A searchable database of over 800 common compound names, formulas, structures, and properties.

Companion Notes Hyperlinked notes and guides for first semester general chemistry. Construction Kits Flash-based kits for building chemical formulas, names, equations, and problem solutions. Articles Featured articles, books, and tutorials. Toolbox Interactive graphing, popup tables, and calculators. Tutorials Index of self-guided tutorials, quizzes, and drills on specific topics. UNC Chemistry Fundamentals. An Interactive Educational Exercise Because of special formatting tags needed to display exponents, this site is best viewed with Netscape 3.0 or higher.

If needed, use the link under Useful Materials to download Netscape About the Chemistry Fundamentals Course. Nonrenewable and Renewable Energy Resources : QUEST. There are nine major areas of energy resources.

They fall into two categories: nonrenewable and renewable. Nonrenewable energy resources, like coal, nuclear, oil, and natural gas, are available in limited supplies. This is usually due to the long time it takes for them to be replenished. Potential Energy. Kinetic and Potential Energy. Kinetic energy is energy possessed by a body by virtue of its movement.
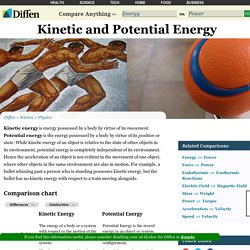
Potential energy is the energy possessed by a body by virtue of its position or state. While kinetic energy of an object is relative to the state of other objects in its environment, potential energy is completely independent of its environment. Hence the acceleration of an object is not evident in the movement of one object, where other objects in the same environment are also in motion. For example, a bullet whizzing past a person who is standing possesses kinetic energy, but the bullet has no kinetic energy with respect to a train moving alongside.
Physical and Chemical Properties of Matter. We are all surrounded by matter on a daily basis.
Anything that we use, touch, eat, etc. is an example of matter. Matter can be defined or described as anything that takes up space, and it is composed of miniscule particles called atoms. The Kinetic Theory of Matter: Definition & The Four States of Matter. Everything on Earth is made of matter, but that matter isn't always the same.
Matter can exist in four different phases, and the kinetic theory of matter helps us understand the differences between those phases. Explore our library of over 10,000 lessons Click "next lesson" whenever you finish a lesson and quiz. Got It You now have full access to our lessons and courses. You're 25% of the way through this course! TED-Ed and Periodic Videos. Kids science: Periodic Table of Elements. Science >> Chemistry for Kids The Periodic Table is a way of listing the elements.
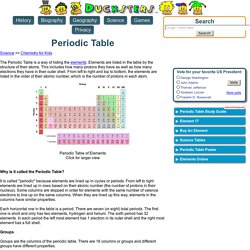
Elements are listed in the table by the structure of their atoms. This includes how many protons they have as well as how many electrons they have in their outer shell. From left to right and top to bottom, the elements are listed in the order of their atomic number, which is the number of protons in each atom. Periodic Table of Elements Click for larger view Why is it called the Periodic Table? The Periodic Table of Elements. Matter: Chemical vs. Physical Changes. It is important to understand the difference between chemical and physical changes.

Some changes are obvious, but there are some basic ideas you should know. Physical changes are usually about states and physical states of states. Chemical changes happen on a molecular level when you have two or more molecules that interact. Chemical changes happen when atomic bonds are broken or created during chemical reactions. When you step on a can and crush it, you have forced a physical change. When you melt an ice cube (H2O), you have a physical change because you add energy. Chemical changes happen on a much smaller scale.
Melting a sugar cube is a physical change because the substance is still sugar. Kids.Net.Au - Dictionary > Definition: law of conservation of mass. Law of Conservation of Energy and Mass. What is chemical energy? Science Classroom. Science Discovery Days (Student worksheets provided) Scientific Method Unit & Safety Rules (Unit notes, worksheets, and lab ideas provided) Consumer Challenge (Student worksheets provided) Old Wives Tales Investigation (Student worksheets provided) Silly Science (Classification) (Student worksheet provided) Mystery Bags Film Canister Fun Bioglyphs (Student worksheets provided) Pottery Pieces Innovative Inventions - Internet project (Sites from the Kid Zone) (Student worksheet provided) Inventor's Challenge - Internet project (Sites from the Kid Zone) (Student worksheet provided) A Journey Through Time -Internet project (Sites from the Kid Zone) (Student worksheet provided) Science A to Z Puzzle (Student worksheet provided) Super Scientist Challenge (Student worksheets provided) Also check out ...
Metric Mania - An assortment of lessons and links for the metric system! | Back to top | Teaching the Scientific Method. How to introduce students to the scientific method Students, and sometimes even teachers, often think scientists only use the scientific method to answer science-related questions.
In fact, you can apply the scientific method to almost any problem. The key is to use the elements (steps) to reduce bias and help come to a solution to the problem. The scientific method is the standard in the laboratory, but don’t be fooled by the name. It is also used beyond the laboratory to solve everyday mysteries and problems. One size does not fit all The scientific method consists of a number of different steps, but the order in which we apply the steps can vary. While you can reorder the steps of the scientific method, it is important to apply all the steps to reduce the impact of personal bias. Steps common to versions of the scientific method. Questions and Answers. Elements, Compounds & Mixtures. Elements and Compounds. Elements, Atoms, and Molecules For a more advanced list of resources on atoms, elements and compounds Elements are substances that cannot be separated into simpler substances.