

TED-Ed and Periodic Videos. 10 Incredible Chemical Reaction GIFs Explained. We encounter thousands of chemical reactions every day: plants use them in photosynthesis, metals rust over time, and combustion reactions provide us with heat and light, among thousands of other daily uses.
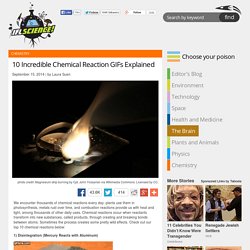
Chemical reactions occur when reactants transform into new substances, called products, through creating and breaking bonds between atoms. Sometimes the process creates some pretty wild effects. Periodic Table of Storytelling. Free kids chemistry experiment. The Six Types of Chemical Reaction.
All chemical reactions can be placed into one of six categories.

Here they are, in no particular order: 1) Combustion: A combustion reaction is when oxygen combines with another compound to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. An example of this kind of reaction is the burning of napthalene: 2) Synthesis: A synthesis reaction is when two or more simple compounds combine to form a more complicated one. One example of a synthesis reaction is the combination of iron and sulfur to form iron (II) sulfide: 8 Fe + S8 ---> 8 3) Decomposition: A decomposition reaction is the opposite of a synthesis reaction - a complex molecule breaks down to make simpler ones.
One example of a decomposition reaction is the electrolysis of water to make oxygen and hydrogen gas: 4) Single displacement: This is when one element trades places with another element in a compound. Mg + 2 H2O ---> Mg(OH)2 + H2 Pb(NO3)2 + 2 KI ---> PbI2 + 2 KNO3 HBr + NaOH ---> NaBr + H2O. Combustion Simulation. In a chemical reaction a reactant is consumed during a reaction while a product is formed as a result.
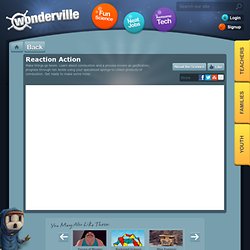
In this game two examples of chemical reactions are highlighted: combustion and gasification. In both cases a hydrocarbon (such as oil, coal or wood) and oxygen are the reactants, while the products vary depending on the process and amount of oxygen. Any product of a chemical reaction that is not the intended product of the reaction is a by-product. In combustion processes, heat is the main intended product, and the chemical compounds that are produced are considered by-products. The by-products produced by combustion depend on the type of reaction and reactants present. There are two types of combustion: complete and incomplete.
Gasification converts hydrocarbons by reacting them at high temperatures with a controlled amount of oxygen and steam. Chemistry is EASY! How do you write electron configurations? Top 10 Mad Science-Worthy Chemistry Experiments. Chemistry is a fascinating science, but it's often taught poorly in today's boring schools.
Here's how chemistry should be taught: by mad scientists! Here's Neatorama's list of the Top 10 Mad Science-Worthy Chemistry Experiments: 1. Briggs-Rauscher Reaction [YouTube Clip] The Briggs-Rauscher reaction is a well known example of oscillating chemical reactions, also known as chemical clocks because the periodicity can be used to tell time. 2. Who'da thunk that Gummy Bear can be so ... violent? [YouTube Clip] 3. Mentos in various carbonated liquids. You've all seen this before. MythBusters explain: Whatever you do, don't eat a mentos then chug a mouthful of diet soda, mmkay? 4. [YouTube Clip] Yes, even elephants need to maintain good dental hygiene, but what kind of toothpaste do they use?
This one's easy to do, all you need is dish soap, hydrogen peroxide, and potassium iodide: Link 5. What happens if you put a grape and nuke it in a microwave? [YouTube Clip] Galvaniccelltypes124CH2012 - home. The ChemCollective: Virtual Lab. Recommended Version 7 Update 55 Select the file according to your operating system from the list below to get the latest Java for your computer.

By downloading Java you acknowledge that you have read and accepted the terms of the end user license agreement <p><span class="termhighlight">In order to optimize your experience and provide you with accurate messages, please enable javascript in your browser for the duration of your Java installation. </span></p> What is Java?