

Chemistry. Ion. An ion (/ˈaɪən, -ɒn/)[1] is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive or negative electrical charge. Ions can be created by both chemical and physical means. In chemical terms, if a neutral atom loses one or more electrons, it has a net positive charge and is known as a cation. If an atom gains electrons, it has a net negative charge and is known as an anion. Chemical Reactions. A thermite reaction using iron(III) oxide. The sparks flying outwards are globules of molten iron trailing smoke in their wake.
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another.[1] Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur. Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration, and rapid reactions are often described as spontaneous, requiring no input of extra energy other than thermal energy.
History Equations. Periodic table. Physics. Various examples of physical phenomena.
Atoms. About Atoms. Parts of an Atom. As of July 1, 2013 ThinkQuest has been discontinued.

We would like to thank everyone for being a part of the ThinkQuest global community: Students - For your limitless creativity and innovation, which inspires us all. Teachers - For your passion in guiding students on their quest. Partners - For your unwavering support and evangelism. Parents - For supporting the use of technology not only as an instrument of learning, but as a means of creating knowledge.
We encourage everyone to continue to “Think, Create and Collaborate,” unleashing the power of technology to teach, share, and inspire. Neutron. The neutron is a subatomic hadron particle that has the symbol n or n0.
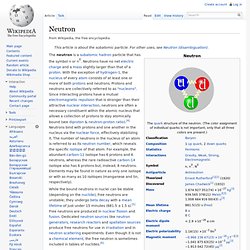
Neutrons have no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen-1, the nucleus of every atom consists of at least one or more of both protons and neutrons. Protons and neutrons are collectively referred to as "nucleons". Since interacting protons have a mutual electromagnetic repulsion that is stronger than their attractive nuclear interaction, neutrons are often a necessary constituent within the atomic nucleus that allows a collection of protons to stay atomically bound (see diproton & neutron-proton ratio).[4] Neutrons bind with protons and one another in the nucleus via the nuclear force, effectively stabilizing it.
Proton. Electron. History[edit] In the early 1700s, Francis Hauksbee and French chemist Charles François de Fay independently discovered what they believed were two kinds of frictional electricity—one generated from rubbing glass, the other from rubbing resin.
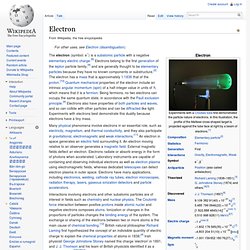
From this, Du Fay theorized that electricity consists of two electrical fluids, vitreous and resinous, that are separated by friction, and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. Electron shell. Periodic table with electron shells In chemistry and atomic physics, an electron shell, also called a principal energy level may be thought of as an orbit followed by electrons around an atom's nucleus.
The closest shell to the nucleus is called the "1 shell" (also called "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with letters used in the X-ray notation (K, L, M, …). Each shell can contain only a fixed number of electrons: The 1st shell can hold up to two electrons, the 2nd shell can hold up to eight (2 + 6) electrons, the 3rd shell can hold up to 18 (2 + 6 + 10) and so on. Biology. History The objects of our research will be the different forms and manifestations of life, the conditions and laws under which these phenomena occur, and the causes through which they have been effected.

The science that concerns itself with these objects we will indicate by the name biology [Biologie] or the doctrine of life [Lebenslehre]. Although modern biology is a relatively recent development, sciences related to and included within it have been studied since ancient times. Natural philosophy was studied as early as the ancient civilizations of Mesopotamia, Egypt, the Indian subcontinent, and China.
However, the origins of modern biology and its approach to the study of nature are most often traced back to ancient Greece.[6] While the formal study of medicine dates back to Hippocrates (ca. 460 BC – ca. 370 BC), it was Aristotle (384 BC – 322 BC) who contributed most extensively to the development of biology. GCSE Bitesize - Biology. Human physiology.