

Oksijen hakkında ansiklopedik bilgi. Elementler içinde çok bol bulunanı olduğu hâlde, eski kimyâcıların gözünden kaçan renksiz, kokusuz ve tatsız bir gaz.
İlk defâ 1774 yılında J.Priestley tarafından, cıva oksidin ısıtılması ile elde edildi. 1781’de Lavoisier, oksijenin, havada bulunan ve yanmayı hâsıl eden bir madde olduğunu bildirdi. Bu maddeye, asit yapısı anlamına gelen oxygenıum ismini verdi. Çünkü Lavoisier, bütün asitlerin oksijen ihtivâ ettiğini sanıyordu. Oksijeni defa 1774 1774 yılı olayları, ölümler, doğumlar ve diğer önemli gelişmeler...Tümünü okumak için linke tıklayınız. Joseph Priestly, cıva oksidinin ısıtılması ile elde edildi. 1781 yılında 1781 yılı olayları, ölümler, doğumlar ve diğer önemli gelişmeler...Tümünü okumak için linke tıklayınız. Lavoisier, oksijenin havada bulunan ve yanmaya etki eden bir madde olduğunu bildirdi. Bulunuşu ...Tümünü okumak için linke tıklayınız. History of atoms. ATOMS (A short history of the knowledge of the atom) Compiled by Jim Walker Originated: Sept. 1988 Latest revision: Nov. 2004 atom n.

A unit of matter, the smallest unit of an element, consisting of a dense, central, positively charged nucleus surrounded by a system of electrons, equal in number to the number of nuclear protons, the entire structure having an approximate diameter of 10-8 centimeter and characteristically remaining undivided in chemical reactions except for limited removal, transfer, or exchange of certain electrons. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. The models they use do not provide an absolute understanding of the atom but only a way of abstracting so that they can make useful predictions about them. The epistemological methods that scientists use provide us with the best known way of arriving at useful science and factual knowledge. The Orbitron: a gallery of atomic orbitals and molecular orbitals.
The Orbitron: a gallery of atomic orbitals and molecular orbitals. C2H2 - Quantum Numbers. Classifying: Calm the Chaos. Hybridization in covalent bonds. From Quantum Numbers to The Periodic Table. A Google image search on the term "atom" will produce two general types of picture: Unfortunately, both of these conventional representations are incorrect (in several ways), and the electronic structure of atoms needs to be understood in terms of the Schrödinger wave equation.
Atoms In stars atomic nuclei are born naked, but their net positive charge – their atomic number Z – attracts the comparatively mass-less electrons to produce neutral atoms. The Net Equation: Your Online Source for Chemistry Solutions. An Atomic Diagram Of Carbon Dioxide, Or Co2, Showing Its Protons, Neutrons And Electrons Including The Carbon And Oxygen Atoms Stock Photo 40318279. All Images Refine Your Search Save to a Lightbox ▼ Please Login...
To organize photos in lightboxes you must first register or login. Registration is Free! Find Similar Images. Clip_image002_thumb26. CO2. CO. Carbon monoxide (CO) is a colorless, odorless, and tasteless gas that is slightly less dense than air.
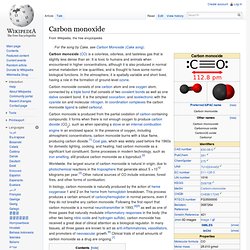
It is toxic to humans and animals when encountered in higher concentrations, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions. In the atmosphere, it is spatially variable and short lived, having a role in the formation of ground-level ozone. Carbon monoxide consists of one carbon atom and one oxygen atom, connected by a triple bond that consists of two covalent bonds as well as one dative covalent bond. It is the simplest oxocarbon, and isoelectronic with the cyanide ion and molecular nitrogen. In coordination complexes the carbon monoxide ligand is called carbonyl. In biology, carbon monoxide is naturally produced by the action of heme oxygenase 1 and 2 on the heme from hemoglobin breakdown.
History[edit] Molecular properties[edit] Bonding and dipole moment[edit] Bond polarity and oxidation state[edit] H2O. Cancel Edit Delete Preview revert Text of the note (may include Wiki markup) Could not save your note (edit conflict or other problem).
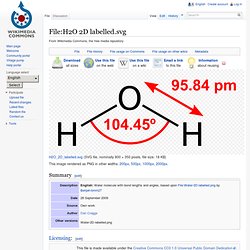
Please copy the text in the edit box below and insert it manually by editing this page. Upon submitting the note will be published multi-licensed under the terms of the CC-BY-SA-3.0 license and of the GFDL, versions 1.2, 1.3, or any later version. See our terms of use for more details. Covalent-water.JPG (JPEG grafiği, 612x432 piksel) Google Google Translate. Google Google Translate.
SCIENCE VIDEO ANIMATION: Biology, Chemistry, Technology, Engineering, Optics, Maths, Astronomy movies. Atomic nucleus. A model of the atomic nucleus showing it as a compact bundle of the two types of nucleons: protons (red) and neutrons (blue).
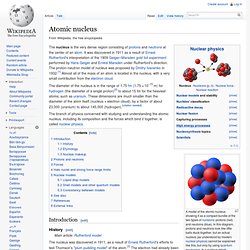
In this diagram, protons and neutrons look like little balls stuck together, but an actual nucleus (as understood by modern nuclear physics) cannot be explained like this, but only by using quantum mechanics. In a nucleus which occupies a certain energy level (for example, the ground state), each nucleon has multiple locations at once. Atomic radius. Diagram of a helium atom, showing the electron probability density as shades of gray.
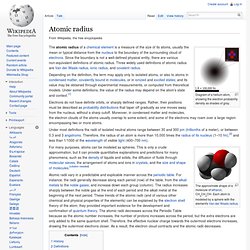
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Atomic nucleus. From New World Encyclopedia Ready A figurative depiction of the helium-4 atom.
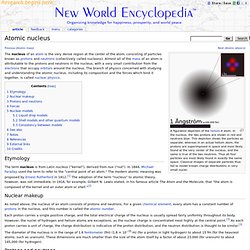
In the nucleus, the two protons are shown in red and neutrons blue. This depiction shows the particles as separate, whereas in an actual helium atom, the protons are superimposed in space and most likely found at the very center of the nucleus, and the same is true of the two neutrons. Thus all four particles are most likely found in exactly the same space. Carbon. Welcome. New 9th Edition (2015) of the Karlsruhe Nuclide Chart now available Through a joint collaboration between Nucleonica GmbH and the JRC, the new 9th Edition (2015) of the Karlsruhe Nuclide Chart has been published.

The 9th edition contains new and updated radioactive decay data on 1644 nuclides not found in the previous 2012 edition. In total, nuclear data on 3992 experimentally observed nuclides are presented.