
Online Books : "TIHKAL" - The Continuation" by Alexander and Ann Shulgin. Yuremamine. Yuremamine is a phytoindole alkaloid which was isolated and identified from the bark of Mimosa tenuiflora in 2005.[1] As a pure compound, yuremamine is a purple amorphous solid. It represents an entirely new family of indole derivatives.
Jump up ^ Vepsäläinen, J. J.; Auriola, S.; Tukiainen, M.; Ropponen, N. & Callaway, J. (2005). "Isolation and characterization of Yuremamine, a new phytoindole". Planta Medica 71 (11): 1049–1053. Muscimol. Muscimol (agarin, pantherine) is the major psychoactive alkaloid present in many mushrooms of the Amanita genus. Muscimol is a potent, selective agonist for the GABAA receptors and displays sedative-hypnotic effects. Chemistry[edit] Muscimol is the psychoactive compound responsible for the effects of Amanita muscaria intoxication. Neurotransmitters and Drugs Chart. Nuciferine. References[edit] Jump up ^ Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Ray AB, Dasgupta B (Sep 1978).
"Psychopharmacological studies on (—)-nuciferine and its Hofmann degradation product atherosperminine". Psychopharmacology (Berl.) 59 (1): 29–33. doi:10.1007/BF00428026. PMID 100809. Aporphine. Aporphine is one of a class of quinoline alkaloids. Tryptamine. Tryptamine is a monoamine alkaloid found in plants, fungi, and animals.
It contains an indole ring structure, and is structurally similar to the amino acid tryptophan, from which it derives its name. Tryptamine is found in trace amounts in the brains of mammals and is believed to play a role as a neuromodulator or neurotransmitter.[2] The tryptamine chemical structure is the backbone for a group of compounds termed collectively tryptamines.
This group includes many biologically active compounds, including neurotransmitters and psychedelic drugs. The concentration of tryptamine in rat brains is about 3.5 pmol/g.[3] Psilocybin. Psilocybin[nb 1] (/ˌsɪləˈsaɪbɪn/ SIL-ə-SY-bin) is a naturally occurring psychedelic compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms.
The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. Baeocystin. Baeocystin was first isolated from the mushroom Psilocybe baeocystis,[1] and later from P. semilanceata,[2] Panaeolus renenosus, Panaeolus subbalteatus, and Copelandia chlorocystis.[3] It was first synthesized by Troxler et al. (1959).[4] Little information exists with regard to human pharmacology, but in the book Magic Mushrooms Around the World, author Jochen Gartz reports being aware of a study in which "10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin.
" Psilocin. Psilocin (also known as 4-OH-DMT, psilocine, psilocyn, or psilotsin), is a substituted tryptamine alkaloid and a serotonergic psychedelic substance.

It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances.[2] The mind-altering effects of psilocin are highly variable and subjective and resemble those of LSD and DMT. Dimethyltryptamine. History[edit] Another historical milestone is the discovery of DMT in plants frequently used by Amazonian natives as additive to the vine Banisteriopsis caapi to make ayahuasca decoctions.
Biosynthesis[edit] Biosynthetic pathway for N,N-dimethyltryptamine This transmethylation mechanism has been repeatedly and consistently proven by radiolabeling of SAM methyl group with carbon-14 (14C-CH3)SAM).[22][20][24][25][26] 5-MeO-DMT. Bufotenin. Bufotenin (5-HO-DMT, N,N-dimethylserotonin), is a tryptamine related to the neurotransmitter serotonin.
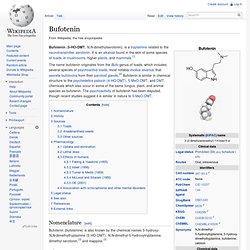
It is an alkaloid found in the skin of some species of toads; in mushrooms, higher plants, and mammals.[1] The name bufotenin originates from the Bufo genus of toads, which includes several species of psychoactive toads, most notably Incilius alvarius, that secrete bufotoxins from their parotoid glands.[2] Bufotenin is similar in chemical structure to the psychedelics psilocin (4-HO-DMT), 5-MeO-DMT, and DMT, chemicals which also occur in some of the same fungus, plant, and animal species as bufotenin. The psychoactivity of bufotenin has been disputed, though recent studies suggest it is similar in nature to 5-MeO-DMT. Nomenclature[edit] N-Methyltryptamine. N-Methyltryptamine (NMT), or methyltryptamine, is a member of the tryptamine chemical class.
It is an alkaloid, probably derived from L-tryptophan, that has been found in the bark, shoots and leaves of several plant species, including Virola, Acacia, Mimosa and Desmanthus often together with the related compounds N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT).[1] It is also synthesized in the human body as a metabolic endproduct of the amino acid L-tryptophan.[2] It was found to be a natural trace component in human urine.[3] NMT has been shown to act as an agonist of the TAAR1, similarly to its relatives tryptamine and N,N-dimethyltryptamine.[7]
Diethyltryptamine. Chemistry[edit] DET is an analogue of the common tryptamine hallucinogen N,N-Dimethyltryptamine or DMT. Pharmacology[edit] The mechanism of action is thought to be serotonin receptor agonism, much like other classic psychedelics.[2] DET is sometimes preferred over DMT because it can be taken orally whereas DMT cannot. This is because the enzyme monoamine oxidase degrades DMT into an inactive compound before it is absorbed. Indole alkaloid. History[edit] The action of some indole alkaloids has been known for ages.
Aztecs used the psilocybin mushrooms which contain alkaloids psilocybin and psilocin. The flowering plant Rauwolfia serpentina which contains reserpine was a common medicine in India around 1000 BC. Africans used the roots of the perennial rainforest shrub Iboga, which contain ibogaine, as a stimulant. An infusion of Calabar bean seeds was given to people accused of crime in Nigeria: its rejection by stomach was regarded as a sign of innocence, otherwise, the person was killed via the action of physostigmine, which is present in the plant and which causes paralysis of the heart and lungs.[3] Consumption of rye and related cereals contaminated with the fungus Claviceps purpurea causes ergot poisoning and ergotism in humans and other mammals. The first indole alkaloid, strychnine, was isolated by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818 from the plants of the Strychnos genus.
Classification[edit] Tryptophan. Tryptophan (IUPAC-IUBMB abbreviation: Trp or W; IUPAC abbreviation: L-Trp or D-Trp; sold for medical use as Tryptan)[2] is one of the 22 standard amino acids and an essential amino acid in the human diet, as demonstrated by its growth effects on rats.
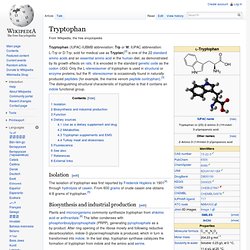
It is encoded in the standard genetic code as the codon UGG. Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the R -stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan).[3] The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. Isolation[edit] The isolation of tryptophan was first reported by Frederick Hopkins in 1901[4] through hydrolysis of casein. Melatonin. Melatonin The hormone can be used as a sleep aid and in the treatment of sleep disorders.
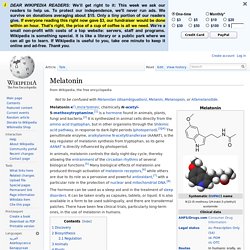
Myristicin. Beta-Carboline. Tryptoline. Pinoline. Pinoline is a methoxylated tryptoline (5-methoxytryptoline) that is produced in the pineal gland during the metabolism of melatonin. Harmala alkaloid. Harmine. Harmine is a fluorescent harmala alkaloid belonging to the beta-carboline family of compounds. It occurs in a number of different plants, most notably the Middle Eastern plant harmal or Syrian rue (Peganum harmala) and the South American vine Banisteriopsis caapi (also known as "yage" or "ayahuasca"). Harmaline. Harmaline is a fluorescent psychoactive indole alkaloid from the group of harmala alkaloids and beta-carbolines. Tranylcypromine. Iproniazid. Phenelzine. Indole alkaloid. Ibogaine. Ibogaine is a naturally occurring psychoactive substance found in plants in the Apocynaceae family such as Tabernanthe iboga, Voacanga africana and Tabernaemontana undulata.
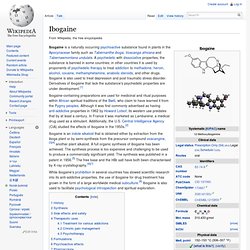
Voacangine. Cannabinoid. Tetrahydrocannabinol. Anandamide. Salvinorin A. Elemicin. Ergotamine. Ergine. Lisuride. Pergolide. Methysergide. Methylergometrine. Lysergic acid hydroxyethylamide. Ergometrine. Ergoline. Lysergic acid diethylamide. Triterpenoid saponins. Mescaline. Gramine. Hordenine. Tropane alkaloid. Hyoscyamine. Atropine. Scopolamine. Nicotine. Arecoline. Piracetam. MDMA. Too much Ecstasy? The man who took 40,000 MDMA pills in 9 years. PiHKAL. MDMA. Ethanol. Thujone. Tiagabine. Ketamine. Phencyclidine. Caffeine. Theophylline. Theobromine. Mirtazapine. Doxylamine. Diphenhydramine. Morphine. Heroin. Codeine. Oxycodone. Cocaine. Amphetamine. Bupropion. Methylphenidate.