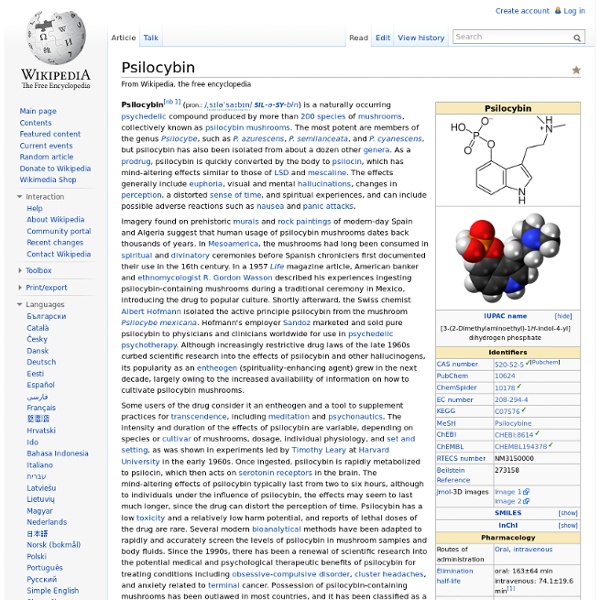
Psilocybin
Psilocybin[nb 1] (/ˌsɪləˈsaɪbɪn/ SIL-ə-SY-bin) is a naturally occurring psychedelic compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms. The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. As a prodrug, psilocybin is quickly converted by the body to psilocin, which has mind-altering effects similar (in some aspects) to those of LSD, mescaline, and DMT. In general, the effects include euphoria, visual and mental hallucinations, changes in perception, a distorted sense of time, and spiritual experiences, and can include possible adverse reactions such as nausea and panic attacks. History[edit] Early[edit] Modern[edit] Albert Hofmann (shown here in 1993) purified psilocybin and psilocin from Psilocybe mexicana in the late 1950s. Occurrence[edit]
Psilocybe cubensis - Encyclopedie
Bestel paddo kweeksets in de online smartshop. Wat is Psilocybe cubensis? Psilocybe cubensis is momenteel een van de meest populaire en verkrijgbare natuurlijke psychedelica. Deze soort is bekend onder verschillende namen waaronder Stropharia cubensis, Stropharia cyanescens of Stopharia caerulaescens, maar de meest gebruikte naam is de Mexicaanse. Meer dan 180 paddenstoelsoorten bevatten de tryptamine alkaloïden psilocine en/of psilocybine. De naam van de soortnaam 'Psilocybe' komt van het Griekse woord psilos (kaal) en kube (hoofd), wat in het Nieuw Latijn tot psilocybe is verbasterd. Geschiedenis Op een archeologische vindplaats in de Non Nak Tha regio in Noord-Thailand zijn botten gevonden van vee (zeboes) waaraan men kon zie hoe ze voor menselijk handelen gebruikt zijn. De introductie van de paddenstoel in de 'moderne wereld' begon toen Gordon Wasson naar het Mazateekse dorpje Huatla de Jimenez kwam, en daar een 'velada' sessie van curandera Maria Sabina meemaakte. Plantkundig Gebruik
An overview on how to find Psilocybin Mushrooms. - Mushroom Hunting and Identification
An overview on how to find Psilocybin MushroomsLast updated 4/17/2010 The reason for writing this guide is that a large percentage of new mushroom hunters go about the process completely backwards. Tromping around, picking a random mushroom, and then asking if it is a psychedelic type is a great waste of time for both the hunter and the friendly folks who frequent these boards trying to help ID them. Randomly picking a mushroom and finding a psychedelic one with no prior knowledge about it is right up there with winning the lottery. Step 1: Know your location. What state are you in? Step 2: Know your mushroom. Now that you’ve got a list of mushrooms that grow in your area, it’s time to put in a little book learning and effort. Important things to note while reading their descriptions:Where do they grow? Armed with all of your new found knowledge, pick a place that you think will yield some likely results and go hunting!. Step 4: Know your camera. Step 6: Know your posting skills.
Psilocybin mushrooms in my area.
Throughout the world the species Panaeolus cinctulus (= P. subbalteatus) and several active species of Gymnopilus grow. Rather than create entries for countries, states and provinces which would then have nothing else listed, it is probably a good assumption that these mushrooms grow in those places not listed. If they are not listed for your area, mentally add it. When researching your mushrooms be aware that many have more than one name. It may be that two names that were thought to be different were shown to describe the same fungus, or that it was moved to a new genus, or a whole host of reasons. For example the mushroom Panaeolus cyanescens was once placed in the genus Copelandia and you will therefore sometimes see it written as Coplandia cyanescens. Amanita muscaria can be found in all 50 states and in most countries.Though Psychoactive, it is not a psilocybin mushroom so it does not appear in this list. 'Sp.' ArkansasGymnopilus sp.Panaeolus cinctulusPsilocybe cubensis Africa Asia
Making Spore Prints
Making Spore Prints by Michael Kuo While a single mushroom spore can't be seen by the naked eye, a pile of many spores can--and the color of a mushroom's spores, seen en masse, is a crucial identification feature. Obtaining a mushroom's "spore print" is therefore an essential step in the identification process. Before going through the nuts and bolts of making a spore print at home, it is worth noting that mushrooms frequently make their own spore prints, in nature. In order to make a spore print at home, you will need to have a relatively mature mushroom. Remove the stem from smaller mushrooms and place the cap, gills or pores downward, on a piece of paper or glass. While some spore prints can appear within a few hours, it's often best to wait overnight, just to be sure. Some field guides advocate using black paper for spore prints, since white prints show up more easily. The color of the spore print is what you will compare with descriptions from field guides and keys.
Related:
Related: