

Functions of state. In contrast, mechanical work and heat are process quantities because their values depend on the specific transition (or path) between two equilibrium states.
History[edit] It is likely that the term “functions of state” was used in a loose sense during the 1850s and 60s by those such as Rudolf Clausius, William Rankine, Peter Tait, William Thomson, and it is clear that by the 1870s the term had acquired a use of its own. In 1873, for example, Willard Gibbs, in his paper “Graphical Methods in the Thermodynamics of Fluids”, states: “The quantities V, B, T, U, and S are determined when the state of the body is given, and it may be permitted to call them functions of the state of the body.” Overview[edit] ). ). When a system changes state continuously, it traces out a "path" in the state space. And the volume as functions of time from time to to time we calculate. Intensive and extensive properties. Physical properties of materials and systems are often described as intensive and extensive properties.
Zeroth law of thermodynamics. First law of thermodynamics. The first law of thermodynamics states that energy can't be created or destroyed, but it can be changed.
The law forms the basis of the principle of conservation of energy. This means that anything that uses energy is changing the energy from one kind of energy to another. For example, exercising changes energy from food into kinetic (motion) energy. Energy cannot be created and never goes away; it just changes its form. People can use the changes and kinetic energy, although this may not be helpful to those not familiar with more complex physics. Thermodynamic cycle. Example of P-V diagram of a thermodynamic cycle.
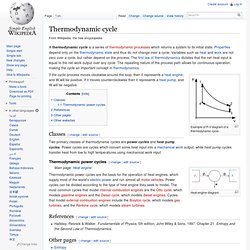
If the cyclic process moves clockwise around the loop, then it represents a heat engine, and W will be positive. If it moves counterclockwise then it represents a heat pump, and W will be negative. Classes[change | edit source] Two primary classes of thermodynamic cycles are power cycles and heat pump cycles. Power cycles are cycles which convert some heat input into a mechanical work output, while heat pump cycles transfer heat from low to high temperatures using mechanical work input.
Thermodynamic power cycles[change | edit source] Heat engine diagram. Thermodynamic power cycles are the basis for the operation of heat engines, which supply most of the world's electric power and run almost all motor vehicles. References[change | edit source] Halliday, Resnick & Walker. Other pages[change | edit source] Ideal gas law. Gas Laws. Heat Transfer.
Adiabatic process. An adiabatic process (/ˌædiəˈbætɪk/; from the Greek privative "a" + "diavaton") is a process that occurs without the transfer of heat or matter between a system and its surroundings.[1][2] A key concept in thermodynamics, adiabatic transfer provides a rigorous conceptual basis for the theory used to expound the first law of thermodynamics.
It is also key in a practical sense, that many rapid chemical and physical processes are described using the adiabatic approximation; such processes are usually followed or preceded by events that do involve heat transfer. Adiabatic processes are primarily and exactly defined for a system contained by walls that are completely thermally insulating and impermeable to matter; such walls are said to be adiabatic. An adiabatic transfer is a transfer of energy as work across an adiabatic wall or sector of a boundary. The adiabatic flame temperature is a virtual quantity. Etymology[edit] The term adiabatic literally means 'not to be passed'. Description[edit] Second law of thermodynamics. The second law of thermodynamics states that the entropy of an isolated system never decreases in the course of every spontaneous (natural) change.
In other words: over time, differences in temperature, pressure, and density tend to even out in a horizontal plane, but not in a vertical plane due to the force of gravity. For example, density and pressure do not even out in a vertical plane, and nor does temperature because gravity acts on individual molecules, and this means molecular kinetic energy interchanges with gravitational potential energy in free path motion between collisions. Entropy for Beginners. In textbooks of thermodynamics the function of state ‘ENTROPY’ can be approached from first principles, making the study of thermodynamics well accessible.
Considerations[edit] In this discussion we will take a closer look at the definition of entropy and the Second Law of Thermodynamics. In classical thermodynamics the entropy is introduced as follows: For any physical system a function of state, S, exists, called “entropy”. For homogeneous closed systems it increases, after a small heat supply δQ at a system temperature T, according to noting that δQ is an inexact differential.
Here we will follow an approach along the lines of the statistical thermodynamics. This introduction begins with the discussion of ‘quantum states’. Third law of thermodynamics. The Third law of thermodynamics says: If an object reaches the absolute zero of temperature (0 K = -273.15C = −459.67 °F), its atoms will stop moving.
The statement is: At absolute zero , the entropy of a perfectly crystalline substance is zero. Experimentally, it is not possible to obtain -273.15°C. It is found that most of the gases either liquify or solidify before reaching such a temperature, gaseous molecules no longer remaining. So far, scientists have been able to get close to, but not exactly, absolute zero.