

EAA presents Study on Aluminium for Safer trucks together with Transport and Environment. Blog Archive » Improving Aluminum Alloy (US) Posted by admin | Comments : (0) With the majority of the Airbus A380 fuselage and 50% of the Boeing 777 made from aluminum, improving its performance is critical.
(Credit Photo @ INS News Agency) EAA presents study on Aluminium for Safer Trucks (April 2012) > Eurometaux. EAA, together with Transport & Environment (T&E), have presented to the press on 20 March a study on the design of tractor fronts for optimised safety and fuel consumption.

The “Safer Trucks” concept brings a valuable contribution to road safety and environment by improving three aspects: passive safety, aerodynamics and safety of vulnerable road users. 03/16 > BE Suède 28 > Découverte d'un nouveau convertisseur catalytique pour le diesel. Energies & environnementDécouverte d'un nouveau convertisseur catalytique pour le diesel.
06/14 > BE Allemagne 575 > Des amortisseurs de vibrations actifs à base d'élastomère. 06/18 > BE Norvège 109 > L'aimant le plus puissant du monde. PhysiqueL'aimant le plus puissant du monde L'IFE [1] (Institut pour les Technologies de l'Energie) a développé l'aimant supraconducteur le plus puissant du monde.
Des travaux de recherche sur les matériaux magnétiques sont conduits depuis plus de 50 ans sur le site de l'IFE. 06/14 > BE Allemagne 575 > Capacité de décharge de 900 mAh/g pour des batteries lithium-soufre. ElectromobilitéCapacité de décharge de 900 mAh/g pour des batteries lithium-soufre L'utilisation du soufre en tant que matériau pour cathode de batterie repose sur de nombreux avantages : densités énergétiques élevées, non-toxicité, coût peu élevé et réserves disponibles en grande quantité.

Or, le soufre possède une faible conductibilité électrique, nécessitant de le combiner à une matrice conductrice pour pouvoir être utilisé dans des applications électrochimiques. Des chercheurs de l'Institut Fraunhofer d'ingénierie des matériaux et des technologies à faisceaux (IWS) de Dresde (Saxe) ont ainsi développé un procédé permettant de faire croître verticalement des nanotubes de carbone sur des substrats métalliques (aluminium, nickel, acier inoxydable).
Le soufre peut alors être introduit au sein des structures précédentes sans ajout de liant ou additif complémentaire. New electrode material could lead to rechargeable sodium batteries. A new electrode material could help make lightweight, powerful rechargeable sodium batteries to replace lithium-ion batteries used in electronics and some electric vehicles.

The material contains widely available iron, instead of the nickel and cobalt commonly used in these electrodes, and enables a similar energy density to electrodes in lithium batteries. Sodium is an attractive candidate to replace lithium in batteries because it’s cheaper and widely available around the world. But building a sodium battery requires redesigning battery technology to accommodate the chemical reactivity and larger size of sodium atoms.
Mine to Magnet. Richard (Rick) Mills Ahead of the Herd As a general rule, the most successful man in life is the man who has the best information The rare earths are a group of 17 elements comprising Scandium, Yttrium, and the Lanthanides.
The Lanthanides are a group of 15 (Cerium, Dysprosium, Erbium, Europium, Gadolinium, Holmium, Lanthanum, Lutetium, Neodymium, Praseodymium, Samarium, Terbium, Thorium, Thulium, Ytterbium) chemically similar elements with atomic numbers 57 through 71, inclusive. Yttrium, atomic number 39, isn’t a lanthanide but is included in the rare earths because it often occurs with them in nature - it has similar chemical properties.
Scandium, atomic number 21 is also included in the group although it usually occurs only in minor amounts. Rare Earths from Mine to Magnet. The rare earths are a group of 17 elements comprising scandium, yttrium and the lanthanides.
The lanthanides are a group of 15 (cerium, dysprosium, erbium, europium, gadolinium, holmium, lanthanum, lutetium, neodymium, praseodymium, samarium, terbium, thorium, hulium, ytterbium) chemically similar elements with atomic numbers 57 through 71, inclusive. Reality Check: First Solar and Tellurium. Is there enough Tellurium to meet the future needs of First Solar (FSLR) and other thin-film CdTe solar sell producers?
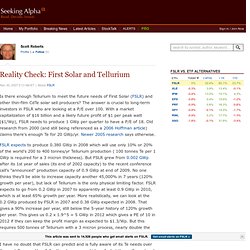
The answer is crucial to long-term investors in FSLR who are looking at a P/E over 100. With a market capitalization of $16 billion and a likely future profit of $1 per peak watt ($1/Wp), FSLR needs to produce 1 GWp per quarter to have a P/E of 18. Old research from 2000 (and still being referenced as a 2006 Hoffman article) claims there's enough Te for 20 GWp/yr. Newer 2005 research says otherwise. FSLR expects to produce 0.380 GWp in 2008 which will use only 10% or 20% of the world's 200 to 400 tonnes/yr Tellurium production ( 100 tonnes Te per 1 GWp is required for a 3 micron thickness). Plusieurs technologies ''vertes'' vont pâtir de tensions sur l'approvisionnement en matières minérales rares. Une liste de quatorze matières minérales critiques a été publiée jeudi 17 juin par la Commission européenne.
Difficilement substituables et produites dans quelques pays, elles sont vitales pour plusieurs technologies environnementales. Graphite: Supply and Demand. A magnified image of large flake graphite using an electron microscope. In 2010 a European Commission included graphite among the 14 materials it considered high in both economic importance and supply risk. The British Geological Survey listed graphite as one of the materials to most likely be in short supply globally. The US has also declared graphite a critical material. The U.S. Graphite: Pencil It In. As a general rule, the most successful man in life is the man who has the best information Sometime between 1500 and 1565 a large graphite deposit was discovered in Cumbria, England.
Because the graphite was extremely pure and solid it could easily be sawed into sticks. The graphite was actually thought to be a form of lead and called plumbago – Latin for lead ore. The Borrowable Mine was soon ordered to be put under armed guard by Queen Elizabeth because the “lead” could be used to line the moulds for making her armies cannonballs. But black marketers managed to smuggle out the graphite for continued use in pencils. Graphite. The mineral / ˈ ɡ r æ f aɪ t / is an allotrope of carbon . Carbon nanotube. Rotating Carbon Nanotube. Allotropes of carbon. Diamond[edit] Diamond is one well known allotrope of carbon. The hardness and high dispersion of light of diamond make it useful for both industrial applications and jewelry. Diamond is the hardest known natural mineral. This makes it an excellent abrasive and makes it hold polish and luster extremely well. Graphene. High-quality graphene is strong, light, nearly transparent and an excellent conductor of heat and electricity.
Its interactions with other materials and with light and its inherently two-dimensional nature produce unique properties, such as the bipolar transistor effect, ballistic transport of charges and large quantum oscillations. At the time of its isolation in 2004,[1] researchers studying carbon nanotubes were already familiar with graphene's composition, structure and properties, which had been calculated decades earlier.
The combination of familiarity, extraordinary properties, surprising ease of isolation and unexpectedly high quality of the obtained graphene enabled a rapid increase in graphene research. Andre Geim and Konstantin Novoselov at the University of Manchester won the Nobel Prize in Physics in 2010 "for groundbreaking experiments regarding the two-dimensional material graphene".[2] Definition[edit]