

Different Types of Corrosion: Pitting Corrosion - Causes and Prevention, WebCorr Corrosion Consulting Services, Corrosion Short Courses and Corrosion Expert Witness. corrosion types, corrosion forms, pipe corrosion, generalized corrosion, pitting corrosio. What causes pitting corrosion?
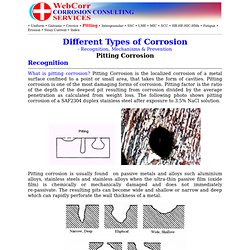
For a defect-free "perfect" material, pitting corrosion is caused by the ENVIRONMENT (chemistry) that may contain aggressive chemical species such as chloride. Chloride is particularly damaging to the passive film (oxide) so pitting can initiate at oxide breaks. The environment may also set up a differential aeration cell (a water droplet on the surface of a steel, for example) and pitting can initiate at the anodic site (centre of the water droplet). For a homogeneous environment, pitting IS caused by the MATERIAL that may contain inclusions (MnS is the major culprit for the initiation of pitting in steels) or defects. In most cases, both the environment and the material contribute to pit initiation. The ENVIRONMENT (chemistry) and the MATERIAL (metallurgy) factors determine whether an existing pit can be repassivated or not. Corrosion and How They Fail".
Passivation. Pickling (metal) Pickling is a metal surface treatment used to remove impurities, such as stains, inorganic contaminants, rust or scale from ferrous metals, copper, and aluminum alloys.[1] A solution called pickle liquor, which contains strong acids, is used to remove the surface impurities.
It is commonly used to descale or clean steel in various steelmaking processes. Many hot working processes and other processes that occur at high temperatures leave a discoloring oxide layer or scale on the surface. In order to remove the scale the workpiece is dipped into a vat of pickle liquor. The primary acid used is hydrochloric acid, although sulfuric acid was previously more common. Hydrochloric acid is more expensive than sulfuric acid, but it pickles much faster while minimizing base metal loss. Carbon steels, with an alloy content less than or equal to 6%, are often pickled in hydrochloric or sulfuric acid. Smooth clean surface (SCS) and eco pickled surface (EPS) are more recent alternatives. 18-8 Stainless steel, 304, 316 Stainless Steel Corrosion. A common misconception about stainless steel is that is not affected by corrosion.

While misleading, the phenomenal success of the metal makes this common belief understandable. One of New York City's most impressive landmarks is the stainless steel clad peak of the Chrysler Building. Built in 1930 of 302 Stainless, a recent inspection revealed no signs of corrosion or loss of thickness. The tallest manmade monument in the US, the St Louis Arch, is entirely clad in 304 stainless steel plates.
Except for cleaning, the stainless exterior of this monument has required no corrosion maintenance. All metals except gold, platinum, and palladium corrode spontaneously To understand the possibility of corrosion in stainless, we must first understand what gives it the ability to resist. To ensure stainless steel is able to "self heal" itself, it is necessary that a finished product, i.e. fasteners, go through a process upon the completion of their manufacturing process. 1. 2. 3. 4.
How To Passivate Stainless Steel Parts. Article From: 10/1/2003 Modern Machine Shop, Terry A.
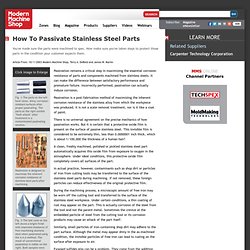
DeBold and James W. Martin Click Image to Enlarge Fig. 1-The parts on the left have clean, shiny, corrosion-resistant surfaces after proper passivating. The parts on the right exhibit "flash attack" after treatment in a contaminated passivating solution. Passivation is designed to maximize the inherent corrosion resistance of stainless steel parts after machining. Fig. 3-The test cone on the left shows a bright finish with improved resistance of free-machining stainless steel when passivated using the A-A-A method. Passivation remains a critical step in maximizing the essential corrosion resistance of parts and components machined from stainless steels. Passivation is a post-fabrication method of maximizing the inherent corrosion resistance of the stainless alloy from which the workpiece was produced.
There is no universal agreement on the precise mechanics of how passivation works. Exposed sulfides also can be a problem. Cleaning First.