

Modelo atómico de Bohr. Niveles de energía. Downloads. DownloadsCompound Interest2015-04-27T22:25:39+00:00 This page contains links to downloads of the high resolution PDFs of infographics on the site.

You will need unarchiving software to extract the files once they have downloaded. If you would prefer to simply view the different graphics, you can do this from the infographics page, the link to which is in the sidebar. Elements | Food Chemistry | Colourful Chemistry | Organic Chemistry | Download Element Infographics The full set of element infographics, containing the graphics for each individual group as well as the timeline of the elements showing discovery dates and countries of discovery. Download Element Infographics – IUPAC Group No. If you’d prefer the element infographics with the correct IUPAC group numbers, rather than those used at GCSE level, then these are the graphics for you.
Download Element Infographics – Teacher Versions Download Food Chemistry Infographics. Beautiful Reactions. Precipitation Chemical Garden Color Change Metal Displacement Crystallization Bubbling Dancing Fluorescent Droplets Smoke.
Atoms and Molecules. There are 114 different kinds of elements that form everything living and nonliving in the world.

Although scientists might discover a few new elements in the future, they are probably very unstable and can only exist in the lab. We use chemical symbols to identify elements. For example, H is for hydrogen, O for oxygen, C for carbon, and S for sulfur. An atom is the basic unit of an element. Different elements have different atoms. A molecule is an assembly of two or more atoms held together by chemical bonds. If we could see the molecules around us, we might see a few molecules on the bottom left.
An atom can lose or gain one or a few electrons, and become positively or negatively charged ions. Moseley Articles. Sept 2012 - This file does not show Greek letters because the HTML has not yet been updated.

It will be soon! By H. G. J. Moseley, M. P. 1024 In the absence of any available method of spectrum analysis, the characteristic types of X radiation, which an atom emits if suitably exited, have hitherto been described in terms of their absorption in aluminum.1 The interference phenomena exhibited by X-rays when scatted by a crystal have now, however, made possible the accurate determination of the frequencies of the various types of radiation. . * Communicated by Prof. Of the internal structure of the atom, and strongly support the views of Rutherford1 and of Bohr.2 Kaye3 has shown that an element excited by a stream of sufficiently fast cathode rays emits its characteristic X radiation. Bohr (II). ¿Cómo convencer a la comunidad científica? ¡Esto es un sinsentido!
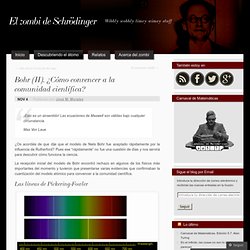
Las ecuaciones de Maxwell son válidas bajo cualquier circunstancia.Max Von Laue ¿Os acordáis de que dije que el modelo de Niels Bohr fue aceptado rápidamente por la influencia de Rutherford? Pues ese “rápidamente” no fue una cuestión de días y nos servirá para descubrir cómo funciona la ciencia. La recepción inicial del modelo de Bohr encontró rechazo en algunos de los físicos más importantes del momento y tuvieron que presentarse varias evidencias que confirmaban la cuantización del modelo atómico para convencer a la comunidad científica. Las líneas de Pickering-Fowler Ejemplo de líneas del espectro del hidrógeno (arriba) y helio (abajo). Eso está muy feo, Pickering. Moseley.jpg (JPEG Image, 430 × 290 pixels) Xxxiii-carnaval-de-quimica-edicic3b3n-arsenico.png (PNG Image, 595 × 595 pixels) La-quimica-es-importante.jpg (JPEG Image, 1600 × 1140 pixels) - Scaled (64%) Química Orgánica « Núcleo virtual de Química. QUÍMICA ORGÁNICA: Principales funciones químicas y sus grupos funcionales.
(En orden de importancia) * Grupo 4 * 4 electrones de valencia * Tetravalente * Electronegatividad intermedia: le permite interactuar con metales y no metales. * 3 Hibridaciones: Fusiones de orbitales para poder ampliar la capacidad de enlaces del carbono. Sp³ / Sp² / Sp CO: Monóxido de carbonoCO₂: Dióxido de carbonoH₂CO₃: Dióxido de carbónicoHCN: ácido cianhídricoHCO¯₃: Ión carbonatoCN¯: Ión cianuro ALCANOS (Cn H₂n ₊₂) Alifático 1) Cadena + larga. 2) Reconocer radicales. 3) Radicales con menor numeración. 4) Colocar en orden alfabético. * Cadena cerrada saturada. 1) Se nombra con la palabra CICLO, (mismas reglas que los alcanos).
ALQUENOS (Cn H₂n) Alifático * Presenta al menos 1 doble enlace.