

Water Research. Lcgc. Alkaline and Acidic Foods Charts (Health Topic) How To Balance Your pH Levels and Find Out if You are Too Acidic. Alkalizing has become a very popular subject recently, and for good reason too!
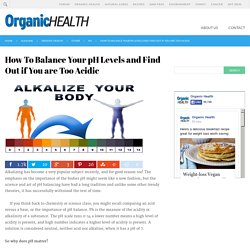
The emphasis on the importance of the bodies pH might seem like a new fashion, but the science and art of pH balancing have had a long tradition and unlike some other trendy theories, it has successfully withstood the test of time. If you think back to chemistry or science class, you might recall comparing an acid versus a base, or the importance of pH balance. Alkaline Diet. Search entire U.S. food database: Enter any parts of food name then hit Return to search.

Green: alkaline or low-acid food; Red: acidic food. Usage Note Foods are color coded: Green for alkaline or low-acid foods and Red for acidic foods. Beware of claims that you can change your blood pH. Health Feature Articles You may have seen advertisements about eating certain foods to change the pH of your blood so that you can prevent cancer, become more youthful, have more energy, or prevent a variety of other diseases.
The pH of any solution can range in number from 0-14. Properties of Some Representative Elements Class Notes. Mendeleeve, a Russian chemist in the 1870's, was the first to successfully partially arrrange the known elements into a chart that we call today the periodic table.

He arranged the elements according to increasing atomic size. When he did this he noticed that certain elements grouped themselves into vertical groupings called families or groups. The periodic table was later rearranged by Mosely. He arranged the elements by increasing atomic number. This is the periodic table we are now familiar with. KS3 Bitesize Science - Acids, bases and metals : Revision. Hydrogen. Hydrogen has an atomic structure consisting of one proton and one electron, making it the lightest of the elements and exists as diatomic molecules.
In the solid state the element has a hexagonal closest packed structure. Hydrogen, with atomic symbol H, is thought to be the most abundant of all elements in the universe. History The name derives from the Greek hydro for "water" and genes for "forming", since it burned in air to form water. It was Henry Cavendish who collected the gas over Mercury in 1766, subjected it to systematic study, and reported his findings in 1766 to the Royal Society. Hydrogen was observed and collected long before it was recognized as a unique gas. Hydrogen's historic use is as an industrial chemical. Sources. Urine/Saliva pH Testing: Another Gimmick to Sell You Something. Some "nutritionists" and other fringe practitioners use a nonsensical urine/saliva test as the basis for evaluating a person's health and prescribing dietary supplements to fix it.
The most visible modern proponent of this test was Gary Martin, who, during the 1980s, operated the American College of Nutripathy, a nonaccredited correspondence school that granted "degrees" in nutrition. One of the school's brochures described nutripathy as "the condensation of most all natural healing and counseling techniques available today . . . . the basics 'boiled' from literally hundreds of different therapies and techniques. " Martin claimed that nutripathic tests could detect "imbalances which, if left to mature, must ultimately manifest as some form of disease process.
" and "discover the root cause of the disease while it is still in the prediagnosable stage. " This urine/saliva test and its associated trappings are utter nonsense. pH-EVALUATION. Course of changes in salivary pH-value... [Oral Health Prev Dent. 2008. Association between salivary pH and metabolic syndrome in women: a cross-sectional study. Dr. Ellie - Kiss Your Dentist Goodbye. Bacteria cause dental disease.

These organisms thrive in a dry, acidic mouth and cause bad breath, plaque build-up, gingivitis, and weakened teeth. Acids from bacteria erode tooth enamel which leads to sensitivity, cavities and discoloration. pH Buffers in the Blood. Acid-Base Equilibria Experiment For information or comments on this tutorial, please contact R.

Frey at gfrey@wuchem.wustl.edu. Key Concepts: Exercise and how it affects the body Acid-base equilibria and equilibrium constants How buffering works Quantitative: Equilibrium Constants Qualitative: Le Châtelier's Principle Le Châtelier's Principle Direction of Equilibrium Shifts Application to Blood pH How Does Exercise Affect the Body? Monitoring your Body's PH levels. Monitoring your Body's PH levels pH: What does it mean?
pH is the abbreviation for potential hydrogen. The pH of any solution is the measure of its hydrogen-ion concentration. Tutorial - Terminology. Page Index Topsy Turvy Decisions.

Grogono's Acid-Base Tutorial. The Washington Manual Nephrology Subspecialty Consult. Urine pH. pH Scale. Ionization of Water: Water molecules exist in equilibrium with hydrogen ions and hydroxide ions. The water equilibrium constant is written as: Kw = [H+] [OH-] Experimentally, it has been found that the concentration of: H+ = OH- = 10-7. Logarithms and pH. The subject of logarithms is best covered in an algebra class, and there are many good explanations on the web: In Chemistry, we work with two types of logarithms. We encounter common (base 10) logarithms in General Chemistry, primarily when dealing with pH. An understanding of natural (base e) logarithms is necessary in some higher-level courses.
Here, we will only discuss common logarithms. Logarithm. The logarithm for a base and a number. Math Skills - Logarithms. Two kinds of logarithms are often used in chemistry: common (or Briggian) logarithms and natural (or Napierian) logarithms. The power to which a base of 10 must be raised to obtain a number is called the common logarithm (log) of the number. The power to which the base e (e = 2.718281828.......) must be raised to obtain a number is called the natural logarithm (ln) of the number. In simpler terms, my 8th grade math teacher always told me: LOGS ARE EXPONENTS!! What did she mean by that? Bogen's Universal Indicator Solution, 4oz for sale. Buy from The Science Company. Kkjc2 Scientific. Talas - Environmental Controls, Instruments & Measures -> pH Testing. pH. The sour taste of lemon juice is a result of it being composed of about 5% to 6% citric acid, an acid with a pH of roughly 2.2.
The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.[1] Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. Measurement of pH for aqueous solutions can be done with a glass electrode and a pH meter, or using indicators. pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications.
History. pH Definition. Definition of pH scales.