

CASP Tools & Checklists. This set of eight critical appraisal tools are designed to be used when reading research, these include tools for Systematic Reviews, Randomised Controlled Trials, Cohort Studies, Case Control Studies, Economic Evaluations, Diagnostic Studies, Qualitative studies and Clinical Prediction Rule.

These are free to download and can be used by anyone under the Creative Commons License. CASP Checklists (click to download) Some Study Designs..... What is a Systematic Review? Frequently there will have been more than one study addressing a particular health question. What is a Randomised Controlled Trial (RCT)? An RCT is a type of interventional or experimental study design. What is a Qualitative study? A qualitative study examines the experiences and beliefs of people from their own perspective. What is a Cohort study? A cohort study, also known as a follow-up or longitudinal study, is another observational study design. STROBE Statement: Available checklists. STROBE checklists Version 4 as published in Oct / Nov 2007!

PRISMA. PEDro scale (English) PEDro. The PEDro scale was last amended on 21 June 1999.

This briefly explains why each item has been included in the PEDro scale. More detail on each item is provided in the PEDro scale training program. 1. eligibility criteria were specified Note on administration: This criterion is satisfied if the report describes the source of subjects and a list of criteria used to determine who was eligible to participate in the study. Explanation: This criterion influences external validity, but not the internal or statistical validity of the trial. 2. subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received) Note on administration: A study is considered to have used random allocation if the report states that allocation was random. Explanation: Random allocation ensures that (within the constraints provided by chance) treatment and control groups are comparable. 3. allocation was concealed.
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. GA Wells, B Shea, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell, Nonrandomised studies, including case-control and cohort studies, can be challenging to implement and conduct.
Assessment of the quality of such studies is essential for a proper understanding of nonrandomised studies. The Newcastle-Ottawa Scale (NOS) is an ongoing collaboration between the Universities of Newcastle, Australia and Ottawa, Canada. It was developed to assess the quality of nonrandomised studies with its design, content and ease of use directed to the task of incorporating the quality assessments in the interpretation of meta-analytic results. COSMIN checklist. The COSMIN checklist The COSMIN checklist contains standards for design requirements and preferred statistical methods of studies on the measurement properties of health measurement instruments.

The checklist can be used to determining if a study on measurement properties meets the standards for good methodological quality. AMSTAR - Assessing the Methodological Quality of Systematic Reviews. Critical Appraisal Checklists - Cardiff University. SURE Critical Appraisal Checklists Over the years SURE team members have worked with a large number of different critical appraisal tools and checklists in our systematic review work and in our teaching.
There are pluses and minuses with all of them. These new SURE checklists owe a debt to all those already developed, particularly the Health Evidence Bulletins Wales (HEBW) checklists, the Critical Appraisal Skills Programme (CASP) checklists and NICE manuals (for clinical and public health guidance development). Many papers have been published over the last ten to fifteen years that indicate the multiplicity of ways in which error and bias can distort the results of papers. We have attempted to capture this work in the SURE checklists, using detailed sub-questions to tease out issues. The checklists are a work in progress and if you use them, we would welcome your feedback (sure@cardiff.ac.uk) on how useful (or otherwise) you found them, along with any suggestions for improvement. 1. 2. Critical Appraisal Tools - Sansom Institute for Health Research.
Critical appraisal is an integral process in Evidence Based Practice.

Critical appraisal aims to identify methodological flaws in the literature and provide consumers of research evidence the opportunity to make informed decisions about the quality of research evidence. Below is a list of critical appraisal tools, linked to the websites where they were developed. iCAHE staff will update this webpage as new critical appraisal tools are published. Healthcare Improvement Scotland have a tutorial on how to best conduct a critical appraisal for those that feel they need a refresher, and the linked YouTube clip gives an introduction to critical appraisal including how to incorpotate evidence into clinical decisions. Please choose a type of study: Randomised Controlled Trials Validation of the PEDro tool: Maher, C. Link to Maher et al. 2003 article (pdf 301KB) Sign50annexc. Critical appraisal tools - Centre for Evidence Based Medicine.
Critical appraisal tools: Newcastle-Ottawa scales for assessing the quality of non-randomised studies (NOS) Critical appraisal tools & checklists: Critical Appraisal Skills Programme (CASP) Critical appraisal tool: NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies - NHLBI, NIH. *CD, cannot determine; NA, not applicable; NR, not reported The guidance document below is organized by question number from the tool for quality assessment of observational cohort and cross-sectional studies.
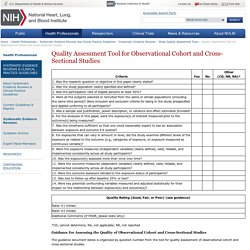
Question 1. Research question Did the authors describe their goal in conducting this research? Is it easy to understand what they were looking to find? Questions 2 and 3. Did the authors describe the group of people from which the study participants were selected or recruited, using demographics, location, and time period? An example would be men over 40 years old with type 2 diabetes who began seeking medical care at Phoenix Good Samaritan Hospital between January 1, 1990 and December 31, 1994. In cohort studies, it is crucial that the population at baseline is free of the outcome of interest. You may need to look at prior papers on methods in order to make the assessment for this question. Question 4. Question 5. Question 6. Question 7.