

The Greenhouse Effect. Acidic Oceans: Why Should We Care? - Perspectives on Ocean Science. The Other Carbon Dioxide Problem. CO2 sinks - Oceans. Fishermen Say Carbon Dioxide Having ‘Really Scary’ Ocean Effect. Healthy oysters on display in Marennes, France Dec. 10 (Bloomberg) -- Jeremy Brown, a fisherman from the Pacific Northwest, is pulling things from the ocean he says are so disturbing that he came to Washington to warn U.S. lawmakers about it.
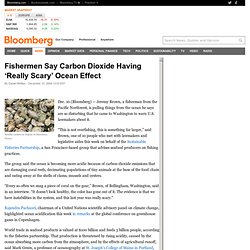
“This is not overfishing, this is something far larger,” said Brown, one of 10 people who met with lawmakers and legislative aides this week on behalf of the Sustainable Fisheries Partnership, a San Francisco-based group that advises seafood producers on fishing practices. The Ocean's Carbon Balance : Feature Articles. After 30 years of research, the question itself hasn’t changed, but the reasoning behind it couldn’t be more different.
Oceanographers started out wanting to know if the ocean was keeping up with the amount of carbon dioxide people are putting into the atmosphere. Instead, they found that people aren’t the only players changing the ocean carbon cycle. Oceans Found to Absorb Half of All Man-Made Carbon Dioxide. John Pickrellfor National Geographic News July 15, 2004 Around half of all carbon dioxide produced by humans since the industrial revolution has dissolved into the world's oceans—with adverse effects for marine life—according to two new studies.
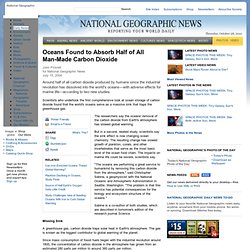
Scientists who undertook the first comprehensive look at ocean storage of carbon dioxide found that the world's oceans serve as a massive sink that traps the greenhouse gas. The researchers say the oceans' removal of the carbon dioxide from Earth's atmosphere has slowed global warming. But in a second, related study, scientists say the sink effect is now changing ocean chemistry. Ocean acidification. NOAA provides evidence for upwelling of corrosive "acidified" water onto the Continental Shelf.
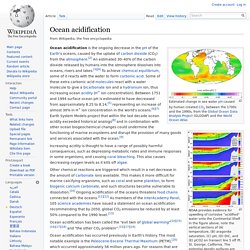
In the figure above, note the vertical sections of (A) temperature, (B) aragonite saturation, (C) pH, (D) DIC, and (E) pCO2 on transect line 5 off Pt. St. George, California. The potential density surfaces are superimposed on the temperature section. What is Acid Rain? Additional Resources Clean Air Status and Trends Network (CASTNET) – CASTNET provides atmospheric data on the dry deposition component of total acid deposition, ground-level ozone and other forms of atmospheric pollution.

National Atmospheric Deposition Program (NADP) – NADP is a network of over 100 federal, state and local government agencies, and private sector entities that collect data on acid deposition, as well as mercury deposition. EPA Clean Air Markets Data and Maps – Provides access to a variety of data associated with emissions trading programs, including trends in emissions and heat input, environmental assessment maps, data sets and reports on acid deposition, facility attributes and contacts, and other file downloads.
Impact of carbon dioxide on water. The Effect of Dissolved Carbon Dioxide on the pH of Water. How does carbon dioxide affect ocean life? Contact: Science Press Packagescipak@aaas.org 202-326-6440American Association for the Advancement of Science As human activities like driving have pumped carbon dioxide into the air, the oceans have absorbed a large portion of this gas.

Richard Feely of the National Oceanic and Atmospheric Administration and his colleagues wanted to know what the effects might be on certain ocean animals that are sensitive to the chemistry of the water they live in. Many mollusks, corals, and single-celled creatures called foraminifera and coccolithophorids use ingredients in seawater to build their shells and other hard parts. Specifically, they pull "carbonate" ions out of the water and make a hard material called "calcium carbonate. " As you might guess from the name, carbon plays an important role in the chemical reactions that allow these animals to make their homes. In parts of the ocean that don't have enough carbonate ions, calcium carbonate shells start to dissolve.
Back to Science for kids. Discovering the Effects of Carbon Dioxide Levels on Marine Life and Global Climate. The causes and effects of climate change have been widely discussed and debated for decades.
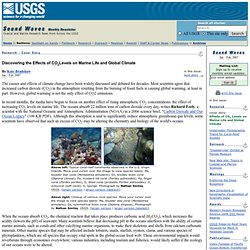
Acid rain. Processes involved in acid deposition (note that only SO2 and NOx play a significant role in acid rain).
Acid clouds can grow on SO2 emissions from refineries, as seen here in Curacao Definition "Acid rain" is a popular term referring to the deposition of wet (rain, snow, sleet, fog, cloudwater, and dew) and dry (acidifying particles and gases) acidic components. Distilled water, once carbon dioxide is removed, has a neutral pH of 7. Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are alkaline. How is carbon dioxide harmful to the environment. Carbon dioxide is a greenhouse gas; too much of it can cause global warming.
It also increases the acidity of the oceans, since it forms carbonic acid when it dissolves in water, and that has a bad effect on some sea life. Well, Carbon dioxide does not harm us directly, but instead it causes green house effect which causes global warming. The reaction between carbon dioxide and water. Carbon dioxide. Carbon dioxide (chemical formula CO2) is a naturally occurring chemical compound composed of 2 oxygen atoms each covalently double bonded to a single carbon atom.
It is a gas at standard temperature and pressure and exists in Earth's atmosphere in this state, as a trace gas at a concentration of 0.039 per cent by volume.[1] The environmental effects of carbon dioxide are of significant interest. Atmospheric carbon dioxide is the primary source of carbon in life on Earth and its concentration in Earth's pre-industrial atmosphere since late in the Precambrian eon was regulated by photosynthetic organisms. Carbon dioxide is an important greenhouse gas; burning of carbon-based fuels since the industrial revolution has rapidly increased the concentration, leading to global warming. It is also a major source of ocean acidification since it dissolves in water to form carbonic acid,[5] which is a weak acid as its ionization in water is incomplete.
History Chemical and physical properties .