

Selenium. Selenium is a chemical element with symbol Se and atomic number 34.
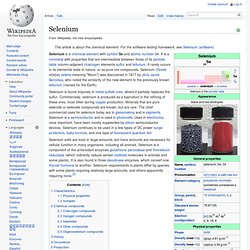
It is a nonmetal with properties that are intermediate between those of its periodic table column-adjacent chalcogen elements sulfur and tellurium. It rarely occurs in its elemental state in nature, or as pure ore compounds. Selenium (Greek σελήνη selene meaning "Moon") was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously known tellurium (named for the Earth). Selenium is found impurely in metal sulfide ores, where it partially replaces the sulfur. Manganese. Manganese is a chemical element, designated by the symbol Mn.
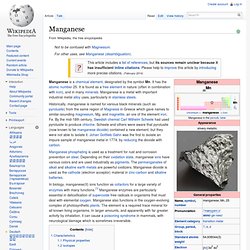
It has the atomic number 25. Boron. Boron is a chemical element with symbol B and atomic number 5.
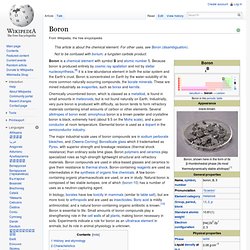
Because boron is produced entirely by cosmic ray spallation and not by stellar nucleosynthesis,[9] it is a low-abundance element in both the solar system and the Earth's crust. Boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. Chromium. Trivalent chromium (Cr(III)) ion is possibly required in trace amounts for sugar and lipid metabolism, although the issue remains in debate.[4] In larger amounts and in different forms, chromium can be toxic and carcinogenic.
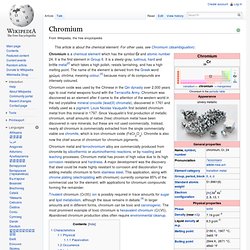
The most prominent example of toxic chromium is hexavalent chromium (Cr(VI)). Abandoned chromium production sites often require environmental cleanup. Characteristics[edit] Physical[edit] Chromium is remarkable for its magnetic properties: it is the only elemental solid which shows antiferromagnetic ordering at room temperature (and below). Cobalt. Cobalt is a chemical element with symbol Co and atomic number 27.
Like nickel, cobalt in the Earth's crust is found only in chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments (cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was later thought by alchemists to be due to the known metal bismuth. Miners had long used the name kobold ore (German for goblin ore) for some of the blue-pigment producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes upon smelting. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the kobold.
Characteristics Compounds. Copper in health. Normal absorption and distribution of copper.
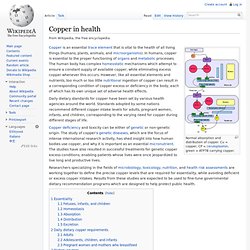
Cu = copper, CP = ceruloplasmin, green = ATP7B carrying copper. Daily dietary standards for copper have been set by various health agencies around the world. Standards adopted by some nations recommend different copper intake levels for adults, pregnant women, infants, and children, corresponding to the varying need for copper during different stages of life. Copper deficiency and toxicity can be either of genetic or non-genetic origin. The study of copper's genetic diseases, which are the focus of intense international research activity, has shed insight into how human bodies use copper, and why it is important as an essential micronutrient. Researchers specializing in the fields of microbiology, toxicology, nutrition, and health risk assessments are working together to define the precise copper levels that are required for essentiality, while avoiding deficient or excess copper intakes.
Fluoride. Fluoride /flʊəraɪd/ is an inorganic anion of fluorine with the chemical formula F−.
It contributes no color to fluoride salts. Fluoride is the main component of fluorite (apart from calcium ions; fluorite is roughly 49% fluoride by mass), and contributes a distinctive bitter taste, but no odor to fluoride salts. Its salts are mainly mined as a precursor to hydrogen fluoride. As it is classified as a weak base, concentrated fluoride solutions will cause skin irritation. Fluoride is the simplest unary fluorine anion, the other being the tentatively investigated trifluorate(2 F—F)(1-) anion. Nomenclature[edit] The systematic name fluoride, the valid IUPAC name, is determined according to the additive nomenclature. Iodine. Iodine is a chemical element with symbol I and atomic number 53.
The name is from Greek ἰοειδής ioeidēs, meaning violet or purple, due to the color of elemental iodine vapor.[2] Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachment to organic compounds have made it a part of many X-ray contrast materials in modern medicine. Iodine has only one stable isotope. A number of iodine radioisotopes are also used in medical applications. Iodine is found on Earth mainly as the highly water-soluble iodide ion I− which concentrates it in oceans and brine pools. Iodine is required by higher animals for synthesizing thyroid hormones, which contain the element. Characteristics[edit] In the gas phase, iodine shows its violet color. I2•PPh3 charge-transfer complexes in CH2Cl2.
Structure and bonding[edit] Structure of solid iodine.