

Key4. Untitled. Chemistry: The Central Science, Chapter 17, Section 5. The solubility of a substance is affected not only by temperature but also by the presence of other solutes.
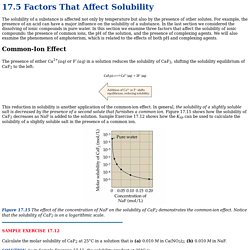
For example, the presence of an acid can have a major influence on the solubility of a substance. In the last section we considered the dissolving of ionic compounds in pure water. In this section we examine three factors that affect the solubility of ionic compounds: the presence of common ions, the pH of the solution, and the presence of complexing agents. We will also examine the phenomenon of amphoterism, which is related to the effects of both pH and complexing agents. Common-Ion Effect. MasteringChemistry: Make Learning Part of the Grade.