

SCIENCE JOURNAL: DENSITY TRIANGLE FOLDABLE COMPLETED. Seven Layer Density Column. Measure 8 ounces of each type of liquid into the 9 ounce portion cups.

You may want to color each of the liquids to make a more dramatic effect in your column. Light Karo syrup is easier to color than dark syrup. The only liquids that you may not be able to color are the vegetable oil and the honey. Start your column by pouring the honey into the cylinder. Now, you will pour each liquid SLOWLY into the container, one at a time. The same amount of two different liquids will have different weights because they have different masses. To test this, you might want to set up a scale and measure each of the liquids that you poured into your column. ** NOTE: The numbers in the table are based on data from manufacturers for each item. The table shows the densities of the liquids used in the column as well as other common liquids (measured in g/cm3 or g/mL). Density is basically how much "stuff" is smashed into a particular area... or a comparison between an object's mass and volume.
Density. Density Tower - Magic with Science. Start your column by pouring the honey into the cylinder.
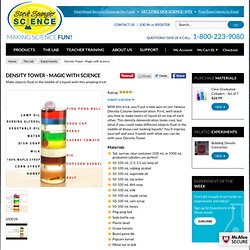
Now, you will pour each liquid SLOWLY into the container, one at a time. It is very important to pour the liquids slowly and into the center of the cylinder. Make sure that the liquids do not touch the sides of the cylinder while you are pouring. It’s okay if the liquids mix a little as you are pouring. The layers will always even themselves out because of the varying densities. The same amount of two different liquids will have different weights because they have different masses. To test this, you might want to set up a scale and measure each of the liquids that you poured into your column. Density is basically how much "stuff" is smashed into a particular area... or a comparison between an object's mass and volume. The same goes for the small objects that you dropped into your density column.
In the materials, we had you grab a bunch of miscellaneous tiny objects. Why do you think these two phenomena happen? Temperature and Density. Are you loving this?
Not loving this? Please consider taking a moment to share your feedback with us. Thanks! Lesson 3.6 Key Concepts Heating a substance causes molecules to speed up and spread slightly further apart, occupying a larger volume that results in a decrease in density. Density—Sink and Float for Liquids. Key Concepts Since density is a characteristic property of a substance, each liquid has its own characteristic density.
The density of a liquid determines whether it will float on or sink in another liquid. A liquid will float if it is less dense than the liquid it is placed in. A liquid will sink if it is more dense than the liquid it is placed in. Density—Sink and Float for Solids. Are you loving this?
Not loving this? Please consider taking a moment to share your feedback with us. Thanks! Chapter 3: Density. Are you loving this?
Not loving this? Please consider taking a moment to share your feedback with us. Thanks! Lesson 3.3 Key Concepts Just like solids, liquids also have their own characteristic density. Summary. Chapter 3: Density.