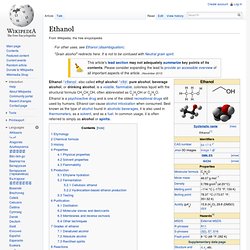
Tetrahydrofuran. Tetrahydrofuran (THF) is an organic compound with the formula (CH2)4O.
The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. THF has an odor similar to acetone. Dimethylformamide. Dimethylformamide is a polar (hydrophilic) aprotic solvent with a high boiling point.
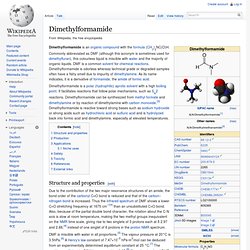
It facilitates reactions that follow polar mechanisms, such as SN2 reactions. Dimethylformamide can be synthesized from methyl formate and dimethylamine or by reaction of dimethylamine with carbon monoxide.[3] Dimethylformamide is reactive toward strong bases such as sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid and is hydrolyzed back into formic acid and dimethylamine, especially at elevated temperatures. Structure and properties[edit] Due to the contribution of the two major resonance structures of an amide, the bond order of the carbonyl C=O bond is reduced and that of the carbon–nitrogen bond is increased.
Thus the infrared spectrum of DMF shows a lower C=O stretching frequency at 1675 cm−1[4] than an unsubstituted C=O bond.
Ethanol. Etymology[edit] Ethanol is the systematic name defined by the International Union of Pure and Applied Chemistry (IUPAC) IUPAC nomenclature of organic chemistry for a molecule with two carbon atoms (prefix "eth-"), having a single bond between them (suffix "-ane"), and an attached -OH group (suffix "-ol").[1] The prefix ethyl was coined in 1834 by the German chemist Justus Liebig.[5] Ethyl is a contraction of the French word ether (any substance that evaporated or sublimated readily at room temperature) and the Greek word ύλη (hyle, substance).[6] The name ethanol was coined as a result of a resolution that was adopted at the International Conference on Chemical Nomenclature that was held in April 1892 in Geneva, Switzerland.[7] Chemical formula[edit]