

Chemical poisoning - general principles of diagnosis and treatment. The diagnosis of chemical poisoning is suspected from a history of exposures resulting in typical clinical syndromes and confirmed by the appropriated medical tests.
There are a series of criteria to be fulfilled to make a confident clinical diagnosis of poisoning by chemicals. The following criteria have to be fulfilled: The subject was fit and well prior to chemical exposures. There is evidence of exposure to the putative chemicals and toxins. The subject initially developed local symptoms which became worse with repeated exposures. The Clinical Picture of Chemical Poisoning It is important to realise that the diagnosis of chemical poisoning relies on recognition of a clinical picture. We have a similar situation with people who have been chemically poisoned. So for a diagnosis of chemical poisoning to be made, not only do the above criteria have to be followed, but a doctor experienced in seeing and treating these new diseases must be able to see the picture.
Past Medical History. Chemical reaction. A thermite reaction using iron(III) oxide.
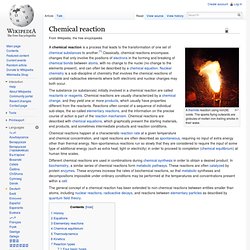
The sparks flying outwards are globules of molten iron trailing smoke in their wake. A chemical reaction is a process that leads to the transformation of one set of chemical substances to another.[1] Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may both occur. Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration, and rapid reactions are often described as spontaneous, requiring no input of extra energy other than thermal energy.
History Equations. Iron. Iron chemical compounds, which include ferrous and ferric compounds, have many uses.
Iron oxide mixed with aluminium powder can be ignited to create a thermite reaction, used in welding and purifying ores. It forms binary compounds with the halogens and the chalcogens. Among its organometallic compounds is ferrocene, the first sandwich compound discovered. Iron plays an important role in biology, forming complexes with molecular oxygen in hemoglobin and myoglobin; these two compounds are common oxygen transport proteins in vertebrates. Iron is also the metal used at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals. Characteristics Mechanical properties The mechanical properties of iron and its alloys can be evaluated using a variety of tests, including the Brinell test, Rockwell test and the Vickers hardness test. Magnesium. In vegetation, magnesium is the metallic ion at the center of chlorophyll, and is, thus, a common additive to fertilizers.[6] Characteristics[edit] Physical properties[edit] Elemental magnesium is a rather strong, silvery-white, light-weight metal (two-thirds the density of aluminium).
It tarnishes slightly when exposed to air, although, unlike the alkali metals, an oxygen-free environment is unnecessary for storage because magnesium is protected by a thin layer of oxide that is fairly impermeable and difficult to remove. Like its lower periodic table group neighbor calcium, magnesium reacts with water at room temperature, though it reacts much more slowly than calcium.
Chemical properties[edit] Magnesium is a highly flammable metal, but, while it is easy to ignite when powdered or shaved into thin strips, it is difficult to ignite in mass or bulk. Oxygen. Blue white glow from an oxygen discharge tube.
Oxygen is an important part of the atmosphere, and is necessary to sustain most terrestrial life as it is used in respiration. However, it is too chemically reactive to remain a free element in Earth's atmosphere without being continuously replenished by the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water. Another form (allotrope) of oxygen, ozone (O 3), strongly absorbs UVB radiation and consequently the high-altitude ozone layer helps protect the biosphere from ultraviolet radiation, but is a pollutant near the surface where it is a by-product of smog. Hydrogen. Hydrogen gas was first artificially produced in the early 16th century, via the mixing of metals with acids.
In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance,[8] and that it produces water when burned, a property which later gave it its name: in Greek, hydrogen means "water-former". Industrial production is mainly from the steam reforming of natural gas, and less often from more energy-intensive hydrogen production methods like the electrolysis of water.[9] Most hydrogen is employed near its production site, with the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market.
Hydrogen is a concern in metallurgy as it can embrittle many metals,[10] complicating the design of pipelines and storage tanks.[11] Properties Combustion 2 H2(g) + O2(g) → 2 H2O(l) + 572 kJ (286 kJ/mol)[note 2] H2 reacts with every oxidizing element. Electron energy levels Main article: Hydrogen atom Phases. Dynamic Periodic Table.