

Your Global GMP Partner
A&C is a global GMP manufacturer of excipients, buffers, process solutions and a select number of APIs. We are a service driven organization finding unique solutions to our customers’ GMP challenges. We provide customized GMP services to pharmaceutical and biopharmaceutical companies.
Magento Development.pdf. ASSURE QUALITY FOCUS SUPPLY CHA - yourglobalgppartner. GOOD MANUFACTURER PRACTICE PACKAGING. Get Information About GMP For Pharmaceutical Excipients .pdf. Ways Ecommerce Website Designing Can Improve Your Business. Out of many industries, e-commerce is the only industry that is growing day by day.
But its growing speed is not constant and, it totally depends on your efforts. Most businesses are intent on growing their revenue faster whether your sales are lagging or are simply okay. To grow your e-commerce business, EcommerceWebsite Designing plays a big role. Through this blog, we will talk about some effective ways of e-commerce website designing for the improvement of your business. Speed optimization of website: Your e-commerce business is only as tough as your website.
Target customers via ads and email: It is to acquire new ones then it more cost-effective to sell to existing customers. Harness the power of the landing page: You need to embrace the landing page if your e-commerce business is serious about achieving rapid growth. Final words This blog is all about eCommerce Website Designing that will help to grow your business. Pinterest.
Privacy Pinterest Today Explore When autocomplete results are available use up and down arrows to review and enter to select.
Touch device users, explore by touch or with swipe gestures. How a Good Website Design Help You Reach Customers: thedigital05 — LiveJournal. A business website is said to be more than just a static marketing piece.
If managed correctly, it could be helpful enough for you to attract more customers. Below mentioned are some ways you can use your website to drive in or attract traffic with fantastic website design, both online and offline. Good SEO Implementation When a website belonging to you is optimized for search, one would be having a better chance of attracting potential customers over there. Research keywords to find out which keywords are searched by the people while they searching online for what to buy or sell.
The addition of these keywords within your titles, tags, and content will be helping get a high reach and engagement over your website. Learn About The Servicing the Life Science Industry A&C – A4 by Your Global GMP Partner. Learn to Know About FDA Registered Manufacturers. What are the main elements of Web Design? – Telegraph. Web designing indeed takes only a short time but a lifetime to master.
No matter what way you choose, there are only two things that can help to make you a professional web design, and these two things are experience and time. Several Web Designing Companies help in branding, marketing, and displaying all the essential information about the company to the customers. Through this blog, you will learn the main elements of web design and, with the help of these elements, you can quickly develop a professional website: Why GMP packaging is Important. Leading and Reliable Buffer Manufacturing Company. Buffer liquids can constitute an expensive bottleneck in bio pharmaceutical downstream processing.

Making these liquids in-house requires regulatory oversight, quality management and dedicated space, equipment and staff for buffer manufacturing, preparation and handling. A&C offers cost effective, flexible and tailored solutions for your buffer outsourcing and cleaning solution needs and always provide full release testing and supporting documentation. At A&C we are experienced in buffer manufacturing and we have the capabilities to manufacture buffers on your behalf in our GMP and ISO 9001:2015 certified facilities*. We manufacture buffers, process solutions, cleaning solutions, custom liquid blends and Water for Injection (WFI), all in compliance with Good Manufacturing Practices (GMP). Our buffers and solutions meet all the current monographs and compendial grades (USP/EP/BP/JP/Multicompendial) where applicable. Novel Excipients. Importance of GMP Quality Control.
With the present electronic detecting gadgets, the dampness of drug clean rooms would now be able to be kept up and observed with resistances as little as one percent.
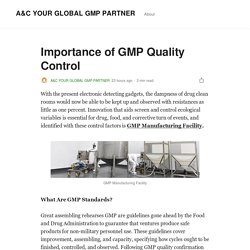
Innovation that aids screen and control ecological variables is essential for drug, food, and corrective turn of events, and identified with these control factors is GMP Manufacturing Facility. What Are GMP Standards? Regulations Of Good Manufacturing Practice by A&C YOUR GLOBAL GMP PARTNER. Best Custom Manufacturing Service. Excipient stable ingredient del - yourglobalgppartner. Why Custom Manufacturing is Important For Manufacturers? – Site Title. During a time where the normal individual can buy a 3D printer and produce anything, the person in question needs, getting a custom manufacturing framework focused on for your business isn’t.
All things considered, the vast majority would prepare a made machine as opposed to having one made especially for their requirements. This is on top of the way that custom arrangements can be more costly than their stock partners. 1. Novel Excipients. Types Of The Manufacturing on Behance. Best Custom Manufacturing Services. Top Trends in The Pharmaceutical Excipients Market – Site Title. Pharmaceutical excipients are more inactive substances other than active ingredients included in drug formulations to serve many purposes.

They can modify drug absorption, pharmacokinetics, and drug stability while overcoming the APIs’ limitations in manufacturing. Excipients are added in drug formulations, and it also improves their taste and appearance while extending their expiration date. What is the role excipients play? The evolution of novel drug delivery systems has brought changes in the regular use of excipients. Presently, they play a variety of different roles in a formulation, and their purpose goes far beyond improving the bulk of the formulation and streamlining the manufacturing process. Types Of Manufacturing Process.pptx. Best Excipient Manufacturer Services. Benefits of Custom Manufacturing.pptx - Zoho Docs. How is Pharma and Healthcare Industry Affecting the Digital Transformation? - A&C Your Global GMP Partner. As there are digital modes for better patient care.
Some people are willing to receive treatment and healthcare using the digital platform. There are new methods of communication and applications available to the users and technologies are being launched. Some points that underline the digital transformation? You have to read further to know exactly. Changing the role of the patient in the treatment Nowadays, digital transformation makes the patient more active in the treatment process. Patients have the best accessibility The availability of the data is humongous, and it is increasing every day. Phenylmethylsulfonyl Fluoride (PMSF) Best PMSF Services in the US. Excipient Selection for Drug Products PowerPoint Presentation, free download - ID:10410037. Download Skip this Video Loading SlideShow in 5 Seconds..
Excipient Selection for Drug Products PowerPoint Presentation Share Presentations Email Sent Successfully. Klusster. As we all know, maintaining a high level of product quality is a series of actions that can endure throughout that product’s life cycle. These actions are often at the core of business optimization strategy, but the quality must start with the manufacture of sale products that adhere to regulatory guidelines. According to the auditor of audit, over the next two to five years, a defined requirement to be compliant with evolving medical device regulations will put companies under a lot of pressure, with Good Manufacturing Practice (GMP) at the top of the table.
What do you by GMP? On a fundamental level, GMP Manufacturing Facility establishes minimum standards for product manufacturing, with the aim being to prevent harm from occurring to the end-users. In most cases, the organization will use the guidelines to limit adulteration and ensure that a high-quality level is present in every product. Let’s discuss some tips to ensure your product GMP quality.
Pin on Novel Excipients. You are signed out Sign in to get the best experience Sign up to see more Continue with Facebook. Good Manufacturing Practices in Employee Training PowerPoint Presentation - ID:10349096. Download Skip this Video Loading SlideShow in 5 Seconds.. Good Manufacturing Practices in Employee Training PowerPoint Presentation Share Presentations Email Sent Successfully Embed Code. Goods Manufacturing Practices For Pharmaceutical Excipients US.
Pharmaceutical excipients manufacturing in the US is essential to drug products' safety and efficacy, as they affect various factors influencing how drugs undertake and interact with the body. These substances, described as those being other than the pharmacologically active drug or prodrug, serve as part of the vehicle transporting the active drug to the site of the body intended to exert its action by preventing the medicine from letting out too early, helping the drug dissolve into particles little enough to reach the bloodstream more quickly, or only making the product look and taste better. Excipients are also integral for pharmaceutical manufacturing. Recipients such as binders, disintegrants, lubricants, coloring agents, and preservatives can determine the final issues' chemical and physical properties. For instance, if diluents are not chosen carefully, they can make the product unstable and lead to manufacturing problems.
The quality management system detailed in NSF involves: Goods Manufacturing Practices for Pharmaceutical Excipients US.pdf. FDA Registered Manufacturers PowerPoint Presentation, free download - ID:10335184. Download Skip this Video Loading SlideShow in 5 Seconds.. Excipient Manufacturer.pdf. GMP Manufacturing Facility & Principles PowerPoint Presentation, free download - ID:10306450. Download Skip this Video. Fundamentals of Quality Control Practice in Pharmaceuticals. QC testing is only designed to test for product attributes that are well-known and understood. These attributes are part of the product specification. However, laboratory testing cannot pick up all the defects the test may not exist, the test may not be sensitive enough, or the test may not be required for that product.
Companies therefore have to rely upon GMP rules and QA systems to prevent these problems from occurring in the first place. Good Manufacturing Practice Packaging. Get ad free downloads and 1 TB of space. FDA Registered Manufacturers Services. Embed Code. ACGGP Novel Excipients Processes. Embed Code. 4shared.com - free file sharing and storage - Document Preview - text. Following Of the Best Practices in Dealing with Novel Excipients. Novel Excipient performs a vital role in bringing new, enhanced, and safer drugs to the pharmaceutical market. There are, however, meaningful challenges in the growth of innovative excipient, mainly because of the lack of globally aligned regulatory mechanisms. Excipients are the main components of any pharmaceutical formulation and crucial for its safe and effective use.
The excipients' functionality is very diverse: They can be used as fillers, lubricants, antioxidants, preservatives, adjuvant, stabilizers, permeation enhancers etc. Excipient Manufacturer. Most Demanding Excipient Manufacturers In UK, PDF, ACGGP. Get ad free downloads and 1 TB of space. Learn More. Process of Tablet Manufacturing and Tablet Packaging. In the pharmaceutical industry, tablet manufacturing is a complex method of making solid pharmaceutical dosage forms. Packaging follows with another round of complicated processes. Meanwhile, unit dose packaging has emerged as the most favored form. GMP Manufacturing Facility. Overview of GMP Manufacturing Facility. Find the Best GMP manufacturing facility in Uk. Embed Code For hosted site: Click the code to copy <div class='visually_embed'><img class='visually_embed_infographic' src=' alt='Find the Best GMP manufacturing facility in Uk | ACGGP' /><div class='visually_embed_cycle'></div><script type='text/javascript' src=' class='visually_embed_script' id='visually_embed_script_1998536'></script><p> From <a href=' For wordpress.com: <div class='visually_embed'><iframe width='1' height='1' style='width: 1px !
The Importance of Good Manufacturing Practices in Food. Behance. Benefits of GMP manufacturing facility to the Pharmaceutical Industry. FDA Registered Manufacturers - A&C Your Global GMP Partner. Pharma Excipient Manufacturer US. GMP Manufacturing Facility. Excipient Manufacturer. GMP Manufacturing Facility. Novel Excipients. Excipient Manufacturer - A&C Your Global GMP Partner. Why GMP? An Explanation of Good Manufacturing Practice.
GMP Manufacturing Facility at A&C Your Global GMP Partner. A&C Your Global GMP Partner and leading GMP Packaging Industry Globally. Custom Manufacturing of Multiple Raw Materials - A&C. Pharma Excipients Manufacturers US of Bio, Pharmaceutica Solutions. With All The Manufacturing Facilities and Standards. Custom Manufacturing of High Purity Solutions. Quality at Pharma Excipients Manufacturers US. Buffer Manufacturing Control the Variations. Cost Effective Buffer Manufacturing Company. Leading Custom Manufacturing Company. What is a GMP Certified Manufacturer? GMP Services Including Custom Manufacturing and Packaging. How to Make Your FDA Audit or Inspection a Success? Custom Manufacturing of Raw Materials at A & C. Leading Customer Manufacturing Firm. Custom Manufacturing of Raw Materials at A & C.
Importance & Benefits of Buffer Manufacturing. Why You Must Know about GMP Guidelines? by A&C YOUR GLOBAL GMP PARTNER. A&C YOUR GLOBAL GMP PARTNER — FDA Registered Manufacturer. A&C Contributes to Potential SARS-COV-2 (Covid-19) Vaccines. Role of Excipient Manufacturer in Pharmaceutical Dosage Form. FDA Registered Manufacturer Focuses on Quality and Supply.
Global Excipient Manufacturer. Best Multicompendial Grade Excipient Manufactures. Why You Must Know about GMP Guidelines? An FDA Registered Facility for Manufacturing Excipients & Raw Material. Trust-able FDA Registered Manufacturers. New GMPs Manufacturing Facility Testing a Key Factor in Manufacturing. A&C YOUR GLOBAL GMP PARTNER. A&C offers Custom Manufacturing Of Excipients And Raw Materials. A&C Offers GMP Services Including Custom Manufacturing and Packaging. GMP Manufacturer of Multicompendial Grade Excipients & Raw Materials. A&C YOUR GLOBAL GMP PARTNER by A&C YOUR GLOBAL GMP PARTNER. An FDA Registered Facility for Manufacturing Excipients & Raw Material. A&C certified by EXCiPACT and Health Canada- why? - A & C. An FDA Registered Facility for Manufacturing Excipients & Raw Material. A&C YOUR GLOBAL GMP PARTNER SERVICES. A&C offers Custom Manufacturing Of Excipients And Raw Materials. A&C YOUR GLOBAL GMP PARTNER.
A&C YOUR GLOBAL GMP PARTNER by A&C YOUR GLOBAL GMP PARTNER.