

Fasciclin domain. In molecular biology, the fasciclin domain (FAS1 domain) is an extracellular domain of about 140 amino acid residues.
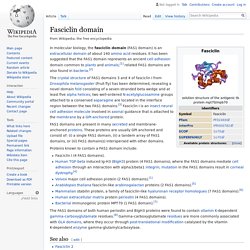
It has been suggested that the FAS1 domain represents an ancient cell adhesion domain common to plants and animals;[1] related FAS1 domains are also found in bacteria.[2] The crystal structure of FAS1 domains 3 and 4 of fasciclin I from Drosophila melanogaster (Fruit fly) has been determined, revealing a novel domain fold consisting of a seven-stranded beta wedge and at least five alpha helices; two well-ordered N-acetylglucosamine groups attached to a conserved asparagine are located in the interface region between the two FAS1 domains.[3] Fasciclin I is an insect neural cell adhesion molecule involved in axonal guidance that is attached to the membrane by a GPI-anchored protein.
FAS1 domains are present in many secreted and membrane-anchored proteins. Proteins known to contain a FAS1 domain include: Cadherins. Identification of a novel gene, DZIP (DAZ-interacting protein), that encodes a protein that interacts with DAZ (deleted in azoospermia) and is expr... - PubMed - NCBI. Wnt signaling pathway. The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors.
Wnt is an acronym in the field of genetics that stands for 'Wingless/Integrated'. The noncanonical planar cell polarity pathway regulates the cytoskeleton that is responsible for the shape of the cell. The noncanonical Wnt/calcium pathway regulates calcium inside the cell. This pathway's clinical importance was demonstrated by mutations that lead to various diseases, including breast and prostate cancer, glioblastoma, type II diabetes and others.[5][6] Encouragingly, in recent years researchers reported first successful use of Wnt pathway inhibitors in mouse models of disease.[7] History and Etymology[edit] The discovery of Wnt signaling was influenced by research on oncogenic (cancer-causing) retroviruses.
Int1 is highly conserved across multiple species, including humans and Drosophila. Akt/PKB signaling pathway. The Akt Pathway, or PI3K-Akt Pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals.
NF-κB. p38 mitogen-activated protein kinases. P38 mitogen-activated protein kinases are a class of mitogen-activated protein kinases (MAPKs) that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy.
Persistent activation of the p38 MAPK pathway in muscle satellite cells (muscle stem cells) due to ageing, impairs muscle regeneration.[1] p38 MAP Kinase (MAPK), also called RK or CSBP (Cytokinin Specific Binding Protein), is the mammalian orthologue of the yeast Hog1p MAP kinase,[2] which participates in a signaling cascade controlling cellular responses to cytokines and stress. Four p38 MAP kinases, p38-α (MAPK14), -β (MAPK11), -γ (MAPK12 / ERK6), and -δ (MAPK13 / SAPK4), have been identified. Similar to the SAPK/JNK pathway, p38 MAP kinase is activated by a variety of cellular stresses including osmotic shock, inflammatory cytokines, lipopolysaccharides (LPS), Ultraviolet light, and growth factors. KLF4 Kruppel like factor 4 [Homo sapiens (human)] - Gene - NCBI. KLF4. In humans, the protein is 513 amino acids with a predicted molecular weight of approximately 55kDa and is encoded by the KLF4 gene.[11] The KLF4 gene is conserved in chimpanzee, rhesus monkey, dog, cow, mouse, rat, chicken, zebrafish, and frog.[12] Interactions[edit] KLF4 can activate transcription by interacting via it N-terminus with specific transcriptional co-activators, such as p300-CBP coactivator family.[13][14][15] Transcriptional repression by KLF4 is carried out by KLF4 competing with an activator for binding to a target DNA sequence (9-12).[16][17][18][19] KLF4 has been shown to interact with CREB-binding protein.[20] It was found that the transcription factor Klf4 present at the promoter of an enzymatic subunit of telomerase (TERT), where it formed a complex with β-catenin.
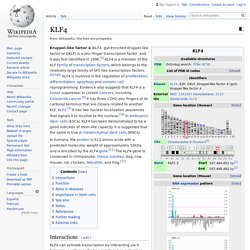
SOX2. SRY (sex determining region Y)-box 2, also known as SOX2, is a transcription factor that is essential for maintaining self-renewal, or pluripotency, of undifferentiated embryonic stem cells.
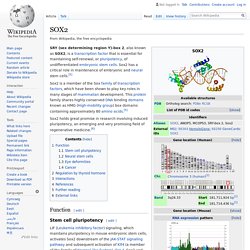
Sox2 has a critical role in maintenance of embryonic and neural stem cells.[5] Sox2 holds great promise in research involving induced pluripotency, an emerging and very promising field of regenerative medicine.[6] Rex1. Rex1 (Zfp-42) is a known marker of pluripotency, and is usually found in undifferentiated embryonic stem cells.
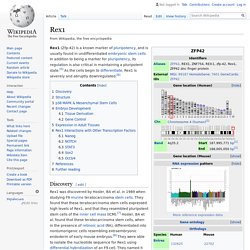
In addition to being a marker for pluripotency, its regulation is also critical in maintaining a pluripotent state.[5] As the cells begin to differentiate, Rex1 is severely and abruptly downregulated.[6] Discovery[edit] Rex1 was discovered by Hosler, BA et al. in 1989 when studying F9 murine teratocarcinoma stem cells. They found that these teratocarcinoma stem cells expressed high levels of Rex1, and that they resembled pluripotent stem cells of the inner cell mass (ICM).[7] Hosler, BA et al. found that these teratocarcinoma stem cells, when in the presence of retinoic acid (RA), differentiated into nontumorigenic cells resembling extraembryonic endoderm of early mouse embryos.[8] They were able to isolate the nucleotide sequence for Rex1 using differential hybridization of an F9 cell.
Oct-4. Oct-4 is a member of the octamer transcription factor family, so named because they bind the octameric (8-unit) DNA nucleotide sequence "ATTTGCAT".[8] Expression and function[edit] Oct-4 transcription factor is initially active as a maternal factor in the oocyte and remains active in embryos throughout the preimplantation period.
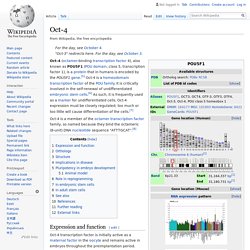
Oct-4 expression is associated with an undifferentiated phenotype and tumors.[9] Gene knockdown of Oct-4 promotes differentiation, demonstrating a role for these factors in human embryonic stem cell self-renewal.[10] Oct-4 can form a heterodimer with Sox2, so that these two proteins bind DNA together.[11] Mouse embryos that are Oct-4 deficient or have low expression levels of Oct-4 fail to form the inner cell mass, lose pluripotency, and differentiate into trophectoderm. Therefore, the level of Oct-4 expression in mice is vital for regulating pluripotency and early cell differentiation since one of its main functions is to keep the embryo from differentiating. Myc. Shinya Yamanaka. Shinya Yamanaka speaking at a lecture on January 14, 2010 Prime Minister of India Narendra Modi visiting Shinya Yamanaka at CiRA, Kyoto University.
Shinya Yamanaka (山中 伸弥, Yamanaka Shin'ya, born September 4, 1962) is a Japanese Nobel Prize-winning stem cell researcher.[2][3][4] He serves as the director of Center for iPS Cell (induced Pluripotent Stem Cell) Research and Application and a professor at the Institute for Frontier Medical Sciences at Kyoto University; as a senior investigator at the UCSF-affiliated J. David Gladstone Institutes in San Francisco, California; and as a professor of anatomy at University of California, San Francisco (UCSF).
Growth differentiation factor. Growth differentiation factors (GDFs) are a subfamily of proteins belonging to the transforming growth factor beta superfamily that have functions predominantly in development.[1] Types[edit] Several members of this subfamily have been described, and named GDF1 through GDF15.
GDF1 is expressed chiefly in the nervous system and functions in left-right patterning and mesoderm induction during embryonic development.[2]GDF2 (also known as BMP9) induces and maintains the response embryonic basal forebrain cholinergic neurons (BFCN) have to a neurotransmitter called acetylcholine, and regulates iron metabolism by increasing levels of a protein called hepcidin.[3][4]GDF3 is also known as "Vg-related gene 2" (Vgr-2). Expression of GDF3 occurs in ossifying bone during embryonic development and in the thymus, spleen, bone marrow brain, and adipose tissue of adults. References[edit] Jump up ^ Herpin A, Lelong C, Favrel P (2004).