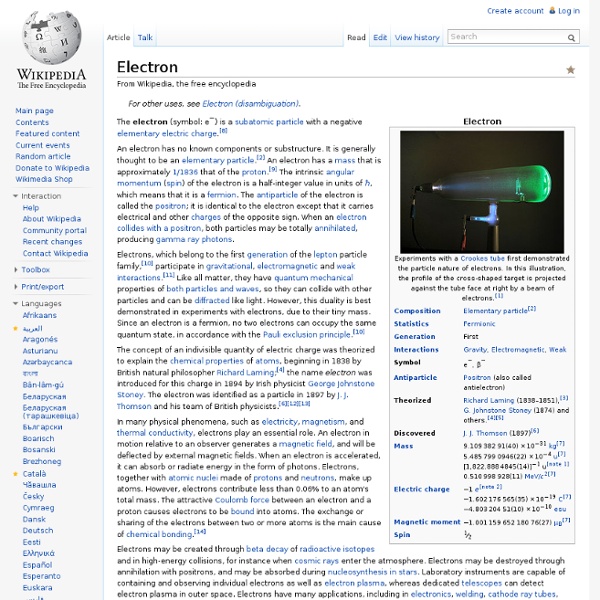
Electron
History[edit] In the early 1700s, Francis Hauksbee and French chemist Charles François de Fay independently discovered what they believed were two kinds of frictional electricity—one generated from rubbing glass, the other from rubbing resin. From this, Du Fay theorized that electricity consists of two electrical fluids, vitreous and resinous, that are separated by friction, and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought of the charge carrier as being positive, but he did not correctly identify which situation was a surplus of the charge carrier, and which situation was a deficit.[19] Discovery[edit] A beam of electrons deflected in a circle by a magnetic field[25] Robert Millikan Atomic theory[edit]
Frequently Asked Questions in Cosmology
Tutorial : Part 1 | Part 2 | Part 3 | Part 4 | Age | Distances | Bibliography | Relativity What is the currently most accepted model for the Universe? The current best fit model is a flat ΛCDM Big Bang model where the expansion of the Universe is accelerating, and the age of the Universe is 13.7 billion years. Back to top. What is the evidence for the Big Bang? The evidence for the Big Bang comes from many pieces of observational data that are consistent with the Big Bang. The darkness of the night sky - Olbers' paradox. Why do we think that the expansion of the Universe is accelerating? The evidence for an accelerating expansion comes from observations of the brightness of distant supernovae. What is quintessence? Quintessence, or the fifth essence, is a fifth element beyond the standard earth, air, fire and water of ancient chemistry. If the Universe is only 14 billion years old, why isn't the most distant object we can see 7 billion light years away? What is the redshift?
Ununoctium
The radioactive ununoctium atom is very unstable, due to its high mass, and since 2005, only three or possibly four atoms of the isotope 294Uuo have been detected.[12] While this allowed for very little experimental characterization of its properties and possible compounds, theoretical calculations have resulted in many predictions, including some unexpected ones. For example, although ununoctium is a member of Group 18, it may possibly not be a noble gas, unlike all the other Group 18 elements.[1] It was formerly thought to be a gas but is now predicted to be a solid under normal conditions due to relativistic effects.[1] History[edit] Unsuccessful synthesis attempts[edit] In late 1998, Polish physicist Robert Smolańczuk published calculations on the fusion of atomic nuclei towards the synthesis of superheavy atoms, including ununoctium.[13] His calculations suggested that it might be possible to make ununoctium by fusing lead with krypton under carefully controlled conditions.[13]
Electromagnetic radiation
The electromagnetic waves that compose electromagnetic radiation can be imagined as a self-propagating transverse oscillating wave of electric and magnetic fields. This diagram shows a plane linearly polarized EMR wave propagating from left to right. The electric field is in a vertical plane and the magnetic field in a horizontal plane. The two types of fields in EMR waves are always in phase with each other with a fixed ratio of electric to magnetic field intensity. Electromagnetic radiation (EM radiation or EMR) is a form of radiant energy, propagating through space via electromagnetic waves and/or particles called photons. In classical physics, EMR is considered to be produced when charged particles are accelerated by forces acting on them. EMR carries energy—sometimes called radiant energy—through space continuously away from the source (this is not true of the near-field part of the EM field). Physics[edit] Theory[edit] Maxwell’s equations for EM fields far from sources[edit]
Radiation
Illustration of the relative abilities of three different types of ionizing radiation to penetrate solid matter. Typical alpha particles (α) are stopped by a sheet of paper, while beta particles (β) are stopped by an aluminium plate. Gamma radiation (γ) is damped when it penetrates lead. Note caveats in the text about this simplified diagram. In electromagnetic radiation (such as microwaves from an antenna, shown here) the term "radiation" applies only to the parts of the electromagnetic field that radiate into infinite space and decrease in intensity by an inverse-square law of power so that the total radiation energy that crosses through an imaginary spherical surface is the same, no matter how far away from the antenna the spherical surface is drawn. Gamma rays, x-rays and the higher energy range of ultraviolet light constitute the ionizing part of the electromagnetic spectrum. Ionizing radiation[edit] Ultraviolet radiation[edit] Main article: Ultraviolet X-ray[edit] Beta radiation[edit]
Related:
Related: