


Welcome to CLEAPSS Royal Society of Chemistry | Advancing excellence in the chemical sciences TecHKnow News - www.techknow.org.uk Details Written by TecHKnow Published: 24 April 2014 The Course is ideal for staff using the Labexpert UK Chemical and Equipment stock control software or who would like to know more about Stock control systems. A Flyer and Application will be available from here shortly Published: 11 April 2014 The number of teachers and support staff working in England's state schools has risen to a record level of 1.3 million people. Read more here...... Published: 10 April 2014 Computer users across the globe are being strongly urged to change all their online passwords, including online banking, email and certain websites because of something called the Heartbleed Bug. This is a popular cryptographic library used to digitally scramble sensitive data as it passes to and from computer servers so that only the service provider and the intended recipients can make sense of it. No pet's names Hackers can find out a lot about you from social media Read more... Written by TecHKnowUK Published: 09 April 2014 In
Free Science Videos and Lectures: Free Education Online is Possible! Scorched hair makes supercapacitors greener Researchers in China have used human hair to make a vital component of energy-storage devices. The discovery could lead to more efficient and environmentally-friendly replacements for traditional batteries. Waste human hair can be turned into carbon flakes that suitable for use in supercapacitor electrodes Many batteries currently in use, such as the lead–acid batteries in cars, are heavy and bulky and rely on hazardous chemicals to store electrical charge. Unfortunately, these carbon materials are usually either difficult to manufacture or derived from fossil fuels. The unique structure of hair creates carbon flakes with an ideal structure for supercapacitor electrodes Human hair has several advantages as a starting material; as well as being cheap and plentiful it naturally contains nitrogen and sulfur, which are retained in the carbon flakes and increase the conductivity of the material.
British Columbia Chemtrail Alert | Monitoring & tracking the criminal toxic chemical spraying of Beautiful British Columbia's land and people Science Daily Ganzfeld effect The ganzfeld effect (from German for “complete field”) or perceptual deprivation, is a phenomenon of perception caused by exposure to an unstructured, uniform stimulation field.[1] It has been most studied with vision by staring at an undifferentiated and uniform field of colour. The visual effect is described as the loss of vision as the brain cuts off the unchanging signal from the eyes. The result is "seeing black"[2] - apparent blindness. It can also elicit hallucinatory percepts in many people, in addition to an altered state of consciousness. Ganzfeld induction in multiple senses is called multi-modal ganzfeld. A related effect is sensory deprivation. A flickering ganzfeld causes geometrical patterns and colors to appear. History[edit] In the 1930s, research by psychologist Wolfgang Metzger established that when subjects gazed into a featureless field of vision they consistently hallucinated and their electroencephalograms changed. See also[edit] References[edit]
Differences between viewing light and dark explain old optical illusion | National Academy of Sciences Astronomers and physicists starting with Galileo noticed centuries ago that when one looks at celestial objects — bright objects on a dark background — they appear to be too large. Now scientists have discovered the brain mechanisms underlying this effect. The findings are reported in the Proceedings of the National Academy of Sciences. Galileo was puzzled by how the appearance of the planets changed depending on whether one looked at them with the naked eye versus a telescope. Now neuroscientist Jens Kremkow at the State University of New York College of Optometry and his colleagues have found this illusion is due to the responses of neurons in the visual pathway and may originate in the very first cells of the pathway — the photoreceptors, which transform light into electricity.
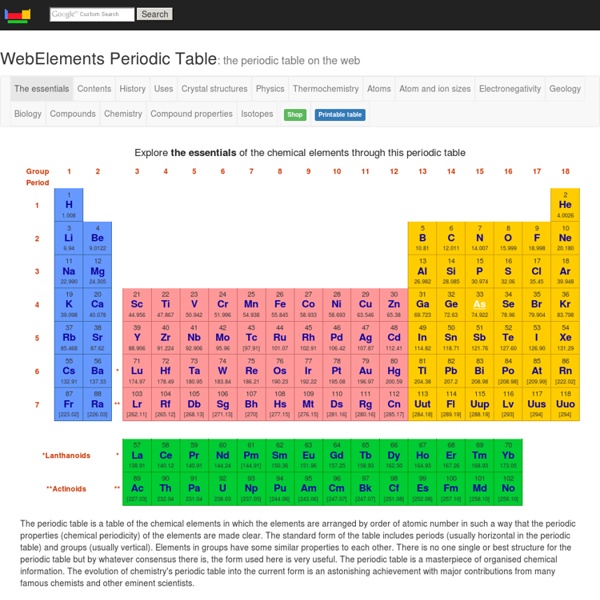
Dont forget the group number tells you how many electrons are in the outer shell
So elements in the same group have similar chemical properies
The period tells you how many shells the atom has.
The smaller number is the atomic number this tells you how many protons are in an atom of that element
The number of protons cannot change. eg Carbon ALWAYS has 6 protons
The larger number is the mass number this tells you how many protons + neutrons are in an atom of that element
(So to work out the number of neutrons take the big number from the little number)
This table shows the mass number with lots of decimal places. In one atom you can't have a quarter of a neutron. This is an average number. because they can have different nubers of nuetrons without affecting its properties (these are called isotopes) by s.burgham Jun 23