


Banach space Definition[edit] or equivalently: All norms on a finite-dimensional vector space are equivalent. Every finite-dimensional normed space is a Banach space.[3] General theory[edit] Linear operators, isomorphisms[edit] For Y a Banach space, the space B(X, Y) is a Banach space with respect to this norm. If X is a Banach space, the space B(X) = B(X, X) forms a unital Banach algebra; the multiplication operation is given by the composition of linear maps. If X and Y are normed spaces, they are isomorphic normed spaces if there exists a linear bijection T : X → Y such that T and its inverse T −1 are continuous. Basic notions[edit] Every normed space X can be isometrically embedded in a Banach space. This Banach space Y is the completion of the normed space X. The cartesian product X × Y of two normed spaces is not canonically equipped with a norm. and give rise to isomorphic normed spaces. If M is a closed linear subspace of a normed space X, there is a natural norm on the quotient space X / M, where
Eigenvalues and eigenvectors Concepts from linear algebra of a linear transformation is scaled by a constant factor when the linear transformation is applied to it: . (possibly negative). The eigenvectors and eigenvalues of a linear transformation serve to characterize it, and so they play important roles in all areas where linear algebra is applied, from geology to quantum mechanics. Consider an matrix A and a nonzero vector of length If multiplying A with (denoted by ) simply scales is called an eigenvector of A, and λ is the corresponding eigenvalue. The following section gives a more general viewpoint that also covers infinite-dimensional vector spaces. In essence, an eigenvector v of a linear transformation T is a nonzero vector that, when T is applied to it, does not change direction. The example here, based on the Mona Lisa, provides a simple illustration. Linear transformations can take many different forms, mapping vectors in a variety of vector spaces, so the eigenvectors can also take many forms. [edit] or . . . . .
Inner product space Geometric interpretation of the angle between two vectors defined using an inner product Definition[edit] Formally, an inner product space is a vector space V over the field F together with an inner product, i.e., with a map that satisfies the following three axioms for all vectors and all scalars Conjugate symmetry: Note that when F = R, conjugate symmetry reduces to symmetry. for F = R; while for F = C, is equal to the complex conjugate[Note 1] of the number Linearity in the first argument: Together with conjugate symmetry, this implies conjugate linearity in the second argument (below). Positive-definiteness: Alternative definitions, notations and remarks[edit] Some authors, especially in physics and matrix algebra, prefer to define the inner product and the sesquilinear form with linearity in the second argument rather than the first. as (the bra–ket notation of quantum mechanics), respectively to be conjugate linear in x rather than y. and is only required to be non-negative. implies x = 0. if
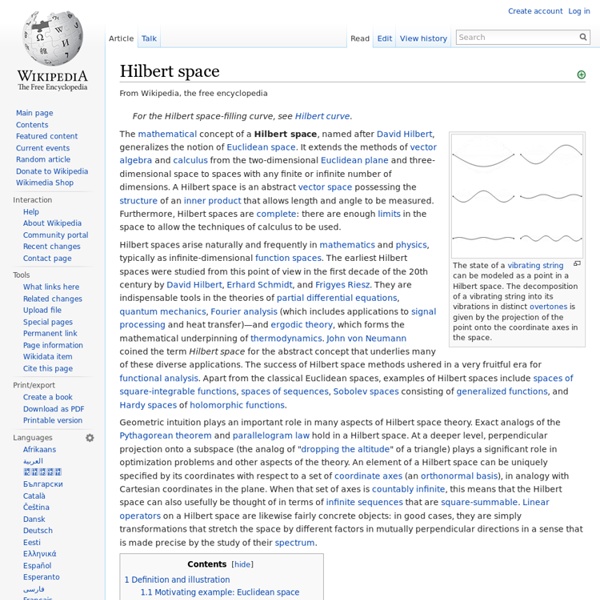
Wave function Mathematical description of the quantum state of a system Comparison of classical and quantum harmonic oscillator conceptions for a single spinless particle. The two processes differ greatly. According to the superposition principle of quantum mechanics, wave functions can be added together and multiplied by complex numbers to form new wave functions and form a Hilbert space. Historical background[edit] In 1905, Albert Einstein postulated the proportionality between the frequency of a photon and its energy ,[11] and in 1916 the corresponding relation between a photon's momentum and wavelength ,[12] where is the Planck constant. , now called the De Broglie relation, holds for massive particles, the chief clue being Lorentz invariance, and this can be viewed as the starting point for the modern development of quantum mechanics. In the 1920s and 1930s, quantum mechanics was developed using calculus and linear algebra. Wave functions and wave equations in modern theories[edit] thus and one obtains
Lp space In mathematics, the Lp spaces are function spaces defined using a natural generalization of the p-norm for finite-dimensional vector spaces. They are sometimes called Lebesgue spaces, named after Henri Lebesgue (Dunford & Schwartz 1958, III.3), although according to the Bourbaki group (Bourbaki 1987) they were first introduced by Frigyes Riesz (Riesz 1910). Lp spaces form an important class of Banach spaces in functional analysis, and of topological vector spaces. Lebesgue spaces have applications in physics, statistics, finance, engineering, and other disciplines. The p-norm in finite dimensions[edit] Illustrations of unit circles in different p-norms (every vector from the origin to the unit circle has a length of one, the length being calculated with length-formula of the corresponding p). The length of a vector x = (x1, x2, ..., xn) in the n-dimensional real vector space Rn is usually given by the Euclidean norm: Definition[edit] Relations between p-norms[edit] When 0 < p < 1[edit]
Five-dimensional space Geometric space with five dimensions Physics[edit] Much of the early work on five-dimensional space was in an attempt to develop a theory that unifies the four fundamental interactions in nature: strong and weak nuclear forces, gravity, and electromagnetism. To explain why this dimension would not be directly observable, Klein suggested that the fifth dimension would be rolled up into a tiny, compact loop on the order of 10-33 centimeters.[1] Under his reasoning, he envisioned light as a disturbance caused by rippling in the higher dimension just beyond human perception, similar to how fish in a pond can only see shadows of ripples across the surface of the water caused by raindrops.[2] While not detectable, it would indirectly imply a connection between seemingly unrelated forces. The main novelty of Einstein and Bergmann was to seriously consider the fifth dimension as a physical entity, rather than an excuse to combine the metric tensor and electromagnetic potential. Polytopes[edit] .
Functional analysis One of the possible modes of vibration of an idealized circular drum head. These modes are eigenfunctions of a linear operator on a function space, a common construction in functional analysis. Normed vector spaces[edit] The basic and historically first class of spaces studied in functional analysis are complete normed vector spaces over the real or complex numbers. More generally, functional analysis includes the study of Fréchet spaces and other topological vector spaces not endowed with a norm. Hilbert spaces[edit] Hilbert spaces can be completely classified: there is a unique Hilbert space up to isomorphism for every cardinality of the orthonormal basis. . Banach spaces[edit] General Banach spaces are more complicated than Hilbert spaces, and cannot be classified in such a simple manner as those. Examples of Banach spaces are -spaces for any real number . on set , then , sometimes also denoted or , has as its vectors equivalence classes of measurable functions whose absolute value's If
Oskar Klein Oskar Benjamin Klein (Swedish: [klajn]; 15 September 1894 – 5 February 1977) was a Swedish theoretical physicist.[1] Biography[edit] Klein was born in Danderyd outside Stockholm, son of the chief rabbi of Stockholm, Gottlieb Klein from Humenné in Slovakia and Antonie (Toni) Levy. He became a student of Svante Arrhenius at the Nobel Institute at a young age and was on the way to Jean-Baptiste Perrin in France when World War I broke out and he was drafted into the military. From 1917, he worked a few years with Niels Bohr in the University of Copenhagen and received his doctoral degree at the University College of Stockholm (now Stockholm University) in 1921. Klein is credited for inventing the idea, part of Kaluza–Klein theory, that extra dimensions may be physically real but curled up and very small, an idea essential to string theory / M-theory. The Oskar Klein Memorial Lecture, held annually at the University of Stockholm, has been named after him. References[edit] External links[edit]