


Carbon subsulfide From Wikipedia, the free encyclopedia Organic compound with the structure S=C=C=C=S Chemical compound Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. Synthesis and structure[edit] C3S2 was discovered by Béla Lengyel,[1] who assigned it an unsymmetrical structure. Lengyel first synthesized this compound by passing carbon disulfide (CS2) vapor through an electric arc with carbon electrodes. Reactions and occurrence[edit] Among its few known reactions, C3S2 reacts with bromine to form the cyclic disulfide.[4] C3S2 polymerizes under applied pressure to give a black semi-conducting solid. In addition, reactions of C3S2 can yield highly condensed sulfur-containing compounds, e.g. the reaction of C3S2 with 2-aminopyridine. References[edit]
Thiamine Chemical compound Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient for humans and animals.[3][4] It is found in food and commercially synthesized to be a dietary supplement or medication.[1][5] Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids.[1] Food sources of thiamine include whole grains, legumes, and some meats and fish.[1][6] Grain processing removes much of the vitamin content, so in many countries cereals and flours are enriched with thiamine.[1] Supplements and medications are available to treat and prevent thiamine deficiency and the disorders that result from it such as beriberi and Wernicke encephalopathy. They are also used to treat maple syrup urine disease and Leigh syndrome. Thiamine supplements are generally well tolerated. Definition[edit] The chemical structure consists of an aminopyrimidine and a thiazolium ring linked by a methylene bridge.
Carbon nitride From Wikipedia, the free encyclopedia Chemical compound made of carbon and nitrogen In organic chemistry, carbon nitrides are compounds consisting only of carbon and nitrogen atoms. Covalent network compounds[edit] Beta carbon nitride - a solid with a formula β-C3N4, which is predicted to be harder than diamond.Graphitic carbon nitride - g-C3N4, with important catalytic and sensor properties.[2]C3N5 - a combined triazole and triazine framework.[3]MCN-12 (C3N6) and MCN-13 (C3N7).[4] Azafullerenes[edit] Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen.[5] Examples include (C59N)2 (biazafullerenyl),[6] C58N2 (diaza[60]fullerene), C57N3 (triaza[60]fullerene) and C48N12. Cyanofullerenes[edit] Cyanofullerenes are a class of modified fullerenes in which cyano- groups are attached to a fullerene skeleton. Cyanogen[edit] Percyanoalkynes, -alkenes and -alkanes[edit] Dicyanopolyynes[edit] Perazidoalkynes, -alkenes and -alkanes[edit] Other compounds[edit]
Exercise Bodily activity that enhances or maintains physical fitness and overall health and wellness Exercise is any bodily activity that enhances or maintains physical fitness and overall health and wellness.[1] It is performed for various reasons, to aid growth and improve strength, develop muscles and the cardiovascular system, hone athletic skills, weight loss or maintenance, improve health,[2] or simply for enjoyment. Many individuals choose to exercise outdoors where they can congregate in groups, socialize, and improve well-being as well as mental health.[3][4] In terms of health benefits, the amount of recommended exercise depends upon the goal, the type of exercise, and the age of the person. Even doing a small amount of exercise is healthier than doing none.[5] Classification An aerobics exercise instructor instructs her class to keep up the pace in the United States. Physical exercises are generally grouped into three types, depending on the overall effect they have on the human body:[6]
Carbomethoxymethylenetriphenylphosphorane From Wikipedia, the free encyclopedia Chemical compound Carbomethoxymethylenetriphenylphosphorane is a chemical compound used in organic syntheses. It contains a phosphorus atom bound to three phenyl groups, and doubly bound to the alpha position of methyl acetate. It undergoes a Wittig reaction.[1] It is used in the Vitamin B12 total synthesis. Production[edit] Carbomethoxymethylenetriphenylphosphorane can be made via a multistep reaction using bromoacetic acid, dicyclohexylcarbodiimide, and triphenylphosphine. [edit] Carbomethoxymethylenetriphenylphosphorane reacts with aldehydes to give a two carbon atom extension. References[edit] Life extension The sale of putative anti-aging products such as nutrition, physical fitness, skin care, hormone replacements, vitamins, supplements and herbs is a lucrative global industry, with the US market generating about $50 billion of revenue each year.[2] Some medical experts state that the use of such products has not been proven to affect the aging process, and many claims of anti-aging medicine advocates have been roundly criticized by medical experts, including the American Medical Association.[2][3][4][5][6] Public opinion[edit] Life extension is a controversial topic due to fear of overpopulation and possible effects on society.[10] Religious people are no more likely to oppose life extension than the unaffiliated,[11] though some variation exists between religious denominations. A Spring 2013 Pew Research poll in the United States found that 38% of Americans would want life extension treatments, and 56% would reject it. Average and maximum lifespans[edit] Diets and supplements[edit]
Covalent organic framework Class of solid chemical substances Covalent organic frameworks (COFs) are a class of materials that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. History[edit] While at University of Michigan, Omar M. The synthesis of 3D COFs has been hindered by longstanding practical and conceptual challenges until it was first achieved in 2007 by Omar M. Structure[edit] Porous crystalline solids consist of secondary building units (SBUs) which assemble to form a periodic and porous framework. Types of porous crystalline solids include zeolites, metal-organic frameworks (MOFs), and covalent organic frameworks (COFs). COFs are another class of porous polymeric materials, consisting of porous, crystalline, covalent bonds that usually have rigid structures, exceptional thermal stabilities (to temperatures up to 600 °C), are stable in water and low densities. COF linkages[edit]
Wikileaks Cables Confirm Existence of Extraterrestrial Life We're creating viewer supported news. Become a member! We’re already halfway through 2013 and the world continues to wake up to the fact that we are not alone in the universe. Just this year, we’ve seen a tremendous step forward regarding UFO disclosure. For more CE articles on the subject of UFOs and extraterrestrials, click HERE. Prior to the recent hearing on UFOs and extraterrestrial life, we’ve had explosive statements made by NASA astronauts and professors. It’s now a fact that UFOs are tracked on radar, performing maneuvers that defy our current understanding of physics. “Behind the scenes, high-ranking Air Force officers are soberly concerned about UFOs. — Former CIA Director Roscoe Hillenkoetter, 1960 The UFO/extraterrestrial phenomenon is extremely top secret; access to this type of documentation and the deeper truth behind it is almost impossible. And another: “I have said in passing there is information about UFOs in Cablegate. – Julian Assange We are living in a unique time.
Carbene radical From Wikipedia, the free encyclopedia Special class of organometallic carbenes Theoretical calculations and EPR studies confirmed their radical-type behaviour and explained the bonding interactions underlying the stability of the carbene radical.[9][10] Stable carbene radicals of other metals are known,[1] but the catalytically relevant cobalt(III)-carbene radicals have thus far only been synthesized as long-lived reactive intermediates.[11][12] Bonding interactions and radical reactivity[edit] The chemical bond present in carbene radicals is surprising in that it possesses aspects of both Fischer and Schrock type carbenes.[1][9][10] As a result, the cobalt carbene radical complexes have discrete radical-character at their carbon atom, thus giving rise to interesting catalytic radical-type reaction pathways. Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs,[1][10] leads the typical radical character of the carbene carbon. See also[edit]
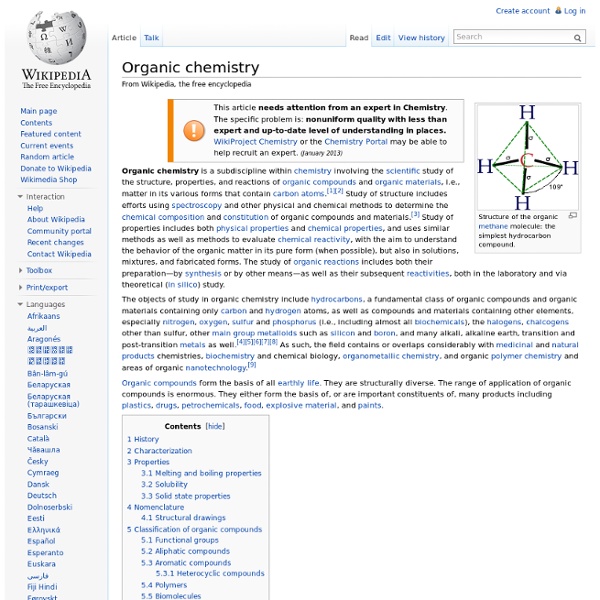
Metabolism Set of chemical reactions in organisms Metabolism (, from Greek: μεταβολή metabolē, "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous.
Biomolecule Molecule that is produced by a living organism The uniformity of both specific types of molecules (the biomolecules) and of certain metabolic pathways are invariant features among the wide diversity of life forms; thus these biomolecules and metabolic pathways are referred to as "biochemical universals"[4] or "theory of material unity of the living beings", a unifying concept in biology, along with cell theory and evolution theory.[5] Types of biomolecules[edit] A diverse range of biomolecules exist, including: Nucleosides and nucleotides[edit] Nucleosides are molecules formed by attaching a nucleobase to a ribose or deoxyribose ring. Nucleosides can be phosphorylated by specific kinases in the cell, producing nucleotides. DNA and RNA structure[edit] DNA structure is dominated by the well-known double helix formed by Watson-Crick base-pairing of C with G and A with T. Saccharides[edit] Monosaccharides are the simplest form of carbohydrates with only one simple sugar. Lignin[edit] Lipid[edit]
Taxonomy Science of classification A taxonomy (or taxonomical classification) is a scheme of classification, especially a hierarchical classification, in which things are organized into groups or types. Among other things, a taxonomy can be used to organize and index knowledge (stored as documents, articles, videos, etc.), such as in the form of a library classification system, or a search engine taxonomy, so that users can more easily find the information they are searching for. Many taxonomies are hierarchies (and thus, have an intrinsic tree structure), but not all are. Originally, taxonomy referred only to the categorisation of organisms or a particular categorisation of organisms. Taxonomy is different from meronomy, which deals with the categorisation of parts of a whole. Etymology[edit] The word was coined in 1813 by the Swiss botanist A. Applications[edit] Mathematically, a hierarchical taxonomy is a tree structure of classifications for a given set of objects. History[edit] Computing[edit]
Diazonium compound Group of organonitrogen compounds Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity[edit] Arenediazonium cations and related species[edit] According to X-ray crystallography the C−N+≡N linkage is linear in typical diazonium salts. The N+≡N bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å,[1] which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. The stability of arenediazonium salts is highly sensitive to the counterion. SN1 and SN2 reactions do not occur. Alkanediazonium cations and related species[edit] Alkanediazonium salts are synthetically unimportant due to their extreme and uncontrolled reactivity toward SN2/SN1/E1 substitution.
Biogenic substance Product made by or of life forms A biogenic substance is a product made by or of life forms. While the term originally was specific to metabolite compounds that had toxic effects on other organisms,[1] it has developed to encompass any constituents, secretions, and metabolites of plants or animals.[2] In context of molecular biology, biogenic substances are referred to as biomolecules. They are generally isolated and measured through the use of chromatography and mass spectrometry techniques.[3][4] Additionally, the transformation and exchange of biogenic substances can by modelled in the environment, particularly their transport in waterways.[5] The observation and measurement of biogenic substances is notably important in the fields of geology and biochemistry. History of discovery and classification[edit] In the 1930s German chemist Alfred E. In the environment[edit] Hydroecology[edit] Geological sites[edit] Measurement[edit] Applications[edit] Anti-fouling paints[edit] Examples[edit]