

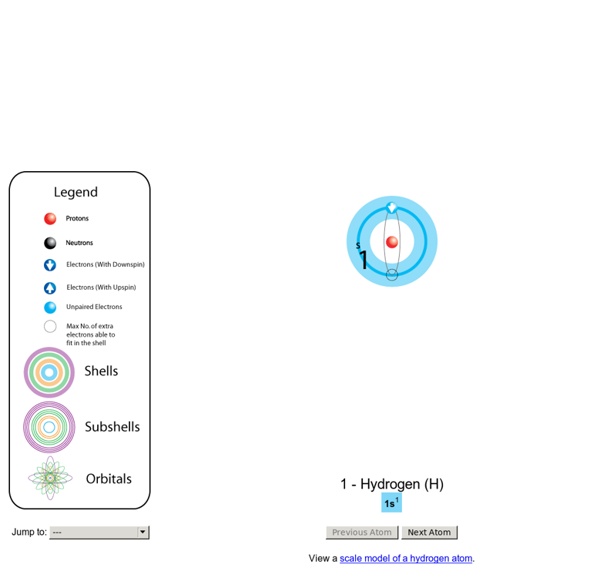
Build an Atom - Phet Topics Atoms Atomic Structure Isotope Symbols Atomic Nuclei Description Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Sample Learning Goals Past Papers This website uses cookies to improve your experience. Please either accept the cookies, or find out how to remove them. Accept Conductive Polymers Lists of Nobel Prizes and Laureates Conductive Polymers Play the Conductive Valley Game About the game Find out what you can use conductive polymers for in the future by furnishing a house! Atomic Orbitals Electron orbitals are the probability distribution of an electron in a atom or molecule. 10 April 2001: A minor update to Orbital Viewer has been posted. The electron orbitals presented here represent a volume of space within which an electron would have a certain probability of being based on particular energy states and atoms. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher energy state, the shapes become lobes and rings, due to the interaction of the quantum effects between the different atomic particles. In addition to technical merits, they make pretty pictures.
Past Papers This website uses cookies to improve your experience. Please either accept the cookies, or find out how to remove them. Accept <div class="js-msg"><p>This website works best with JavaScript switched on. UNC Chemistry Fundamentals An Interactive Educational Exercise Because of special formatting tags needed to display exponents, this site is best viewed with Netscape 3.0 or higher. If needed, use the link under Useful Materials to download Netscape About the Chemistry Fundamentals Course This exercise is designed for anyone who wants an introduction or review of the fundamentals of chemistry that will be used in freshman level chemistry classes.
Past Papers This website uses cookies to improve your experience. Please either accept the cookies, or find out how to remove them. Accept <div class="js-msg"><p>This website works best with JavaScript switched on. <a href=" enable JavaScript</a></p></div> skip to content Chemistry Virtual Textbook Acid-base chemistry can be extremely confusing, particularly when dealing with weak acids and bases. This set of lessons presents an updated view of the Brønsted-Lowry theory that makes it easy to understand answers to common questions: What's the fundamental difference between a strong acid and a weak acid? Can acid A neutralize base B? Why are some salts acidic and others alkaline? How do buffers work?