


Six Thinking Hats Six Thinking Hats is a book by Edward de Bono which describes a tool for group discussion and individual thinking involving six colored hats. "Six Thinking Hats" and the associated idea parallel thinking provide a means for groups to plan thinking processes in a detailed and cohesive way, and in doing so to think together more effectively.[2] Underlying principles[edit] The premise of the method is that the human brain thinks in a number of distinct ways which can be deliberately challenged, and hence planned for use in a structured way allowing one to develop tactics for thinking about particular issues. de Bono identifies six distinct directions in which the brain can be challenged. Since the hats do not represent natural modes of thinking, each hat must be used for a limited time only. A compelling example presented is sensitivity to "mismatch" stimuli. Six distinct directions are identified and assigned a color. Managing Blue - what is the subject? Strategies and programs[edit]
Science » Explore the 5 E’s of Science Engage These lessons mentally engage the students with an event or question. Engagement activities help students to make connections with what they know and can do. What the Teacher Does Creates interestGenerates curiosityRaises questionsElicits responses that uncover what the students know or think about the concept/topic What the Student Does Asks questions, such as Why did this happen? Explore Students work with one another to explore ideas through hands-on activities. Encourages the students to work together without direct instruction from the teacherObserves and listens to the students as they interactAsks probing questions to redirect the students’ investigation when necessaryProvides time for students to puzzle through problemsActs as a consultant for students Thinks freely, but within the limits of the activityTests predictions and hypothesisForms new predictions and hypothesesTries alternatives and discusses them with othersRecords observations and ideasSuspends judgment Explain
Science Fair Projects Ideas, High School, Middle School, Grades Collaborative method Group Setup[edit] Deliberate setup of a team—before beginning work—increases the potential for high performance.[citation needed] To do so, the following components of collaboration should be an initial focus: Group models[edit] Four group models are common in collaboration:[1] Chance Collaboration by chance is the most basic model and underlies all four. Acuity Collaboration by acuity establishes a team with balanced skill sets. Interest Collaboration by interest forms a team of persons with similar hobbies, curiosities or careers. Leader Collaboration by leader is a team model where the members are chosen by a leader. Spence's basic rules[edit] Spence identifies[1] seven rules for all collaboration: Look for common ground: find shared values, consider shared personal experiences, pay attention to and give feedback, be yourself and expect the same of others, be willing to accept differences in perception and opinions Katzenbach and Smith's "team basics"[edit] Complementary skills in team members
History of science "New science" redirects here. For the treatise about history, see The New Science. The history of science is the study of the development of science and scientific knowledge, including both the natural sciences and social sciences. (The history of the arts and humanities is termed as the history of scholarship.) Science is a body of empirical, theoretical, and practical knowledge about the natural world, produced by scientists who emphasize the observation, explanation, and prediction of real world phenomena. Historiography of science, in contrast, often draws on the historical methods of both intellectual history and social history. Early cultures[edit] In prehistoric times, advice and knowledge was passed from generation to generation in an oral tradition. The development of writing enabled knowledge to be stored and communicated across generations with much greater fidelity. Ancient Near East[edit] Greco-Roman world[edit] and again: India[edit] China[edit] Lui Hui's Survey of sea island
Science Fair - Project Ideas Below is a list of great ideas for potential science fair projects. Pick something you're interested in and try it out for size. The projects are categorized by their difficulty. If you're not sure about which project to pick, why not take our Quiz to see which project you might be interested in. Also, feel free to take a look at our projects categorized by Subjects. Easy Projects Our easiest set of original projects. Bag Strength In this project, you can find out how much a bag can hold, so you can impress people next time you go to the grocery store. Battery Testing Which brand of batteries will let your CD player run the longest? Bending Light How can we manipulate the light? Bouncing High Predict how high a ball will bounce. Cube Folding Which arrangements of six squares can be folded into a cube? Food and your Heart What food makes your heart race? Interrogation Check out this statistical experiment to test how phrasing questions might affect the answers. M&M Packing Observing Fish Coin Game
» Life Hacks Household Hacker | Official Website Posted by Dylan Hart in Life Hacks on May 10, 2013 Welcome to my new series where I take “simple life hacks” and prove them true or false. We all see life hacks and tips all over the place, but how do we know they really work without testing them? That’s what I am here for! Let’s break down the unscrupulous and make it digestible. I am on facebook here: if you want the play at home game. Posted by Dylan Hart in Life Hacks on April 23, 2013 Quick and Simple life hacks: FOIL EDITION! 2. 3. 4. 5. Posted by Dylan Hart in Life Hacks on April 17, 2013 Add me on FB: is quick and simple life hacks, where we solve your everyday problems with a little MacGyver inspired ingenuity. Want your ice cream to last longer without getting ruined? Accidentally close a tab in Firefox or Chrome? Ice tray leaking a bit? Having trouble cracking open that glass jar? Need to QUICKLY get rid of cooking odor like fish, grease or burned oil? Wine. 1.
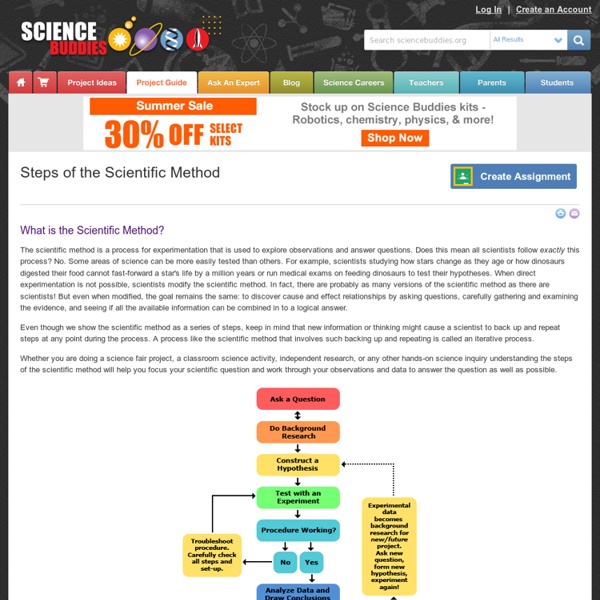
The evolution of scientific knowledge | NOT SO REVIEWS By Bradly Kneisel The Scientific Method We take for granted the information printed in textbooks. 1: Ask a good question 2: Synthesise current scientific opinion surrounding your question 3: Form a hypothesis 4: Predict the logical consequences of the hypothesis 5: Design and perform an experiment to test the hypothesis by collecting data 6: Analyse the data 7: Interpret the data and draw conclusions about them 8: Replicate the experiment 9: Publish results 10: Reproduction and external review Each of these is extremely difficult. On the one hand, the scientific method is useful for research because its application ensures only conclusions that are both empirically evident and logically sound become part of the scientific record. Scientific journals are periodicals of reports of data and results of research experiments, collections of articles that describe application of the scientific method. Journals are structured so as to follow the above sequence of processes. The life of a journal article
List of Science Fair Ideas and Experiments You Can Do. Okay, this is the hardest part of the whole project…picking your topic. But here are some ideas to get you started. Even if you don’t like any, they may inspire you to come up with one of your own. Does music affect on animal behavior? Didn’t see one you like? To download and print this list of ideas CLICK HERE.
About You « Marketing with Heart The bad guys have good marketing and the good guys have bad marketing … we need to change this.” Tad Hargrave Who is this for ? You are an Holistic Practitioner or a Conscious Entrepreneur. You are connected to your Higher Purpose and care deeply about humanity and the planet we live on. You are not motivated by the money but you do want to channel your soul purpose into a consistent income. And of course, you are an active advocate of the “Quadruple Bottom Line” business model: Spiritually Connected - Ecologically Sustainable - Socially Responsible - Economically Prosperous Maybe you’re an Accupuncturist, a Reiki Master or an Energy Healer, a Vibrational Therapist, a Body Worker or Massage Therapist. Perhaps you teach Yoga, Meditation, Tai Chi or Chi Qong or Kung Fu. Maybe you teach Conscious Dance like Five Rythyms, Continuum, Soul Motion, Spirit Weaves, Kundalini Dance, Ecstatic Dance or Biodanza. You get the idea… Your marketing experience so far… This is a good fit for you if…