

Potassium. Most industrial chemical applications of potassium employ the relatively high solubility in water of potassium compounds, such as potassium soaps.
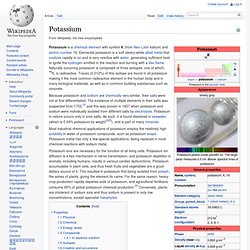
Potassium metal has only a few special applications, being replaced in most chemical reactions with sodium metal. Potassium ions are necessary for the function of all living cells. Potassium ion diffusion is a key mechanism in nerve transmission, and potassium depletion in animals, including humans, results in various cardiac dysfunctions. Potassium accumulates in plant cells, and thus fresh fruits and vegetables are a good dietary source of it. This resulted in potassium first being isolated from potash, the ashes of plants, giving the element its name. Properties[edit] Physical[edit] The flame test of potassium Potassium is the second least dense metal after lithium. Chemical[edit] Potassium atoms have 19 electrons, which is one more than the extremely stable configuration of the noble gas argon.
MgCl2 + 2 K → Mg + 2 KCl Energy levels[edit] Sodium. Sodium is a chemical element with the symbol Na (from Latin: natrium) and atomic number 11.
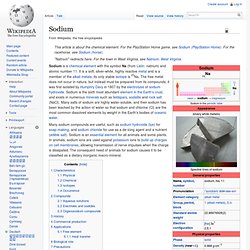
It is a soft, silver-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. The free metal does not occur in nature, but instead must be prepared from its compounds; it was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Sodium is the sixth most abundant element in the Earth's crust, and exists in numerous minerals such as feldspars, sodalite and rock salt (NaCl). Many salts of sodium are highly water-soluble, and their sodium has been leached by the action of water so that sodium and chlorine (Cl) are the most common dissolved elements by weight in the Earth's bodies of oceanic water.
Many sodium compounds are useful, such as sodium hydroxide (lye) for soap-making, and sodium chloride for use as a de-icing agent and a nutrient (edible salt). Characteristics Physical Chemical Isotopes Occurrence Compounds Aqueous solutions. Lithium. The nuclei of lithium verge on instability, since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides.
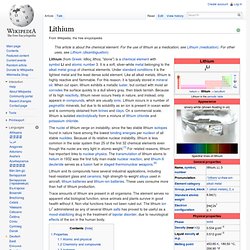
Because of its relative nuclear instability, lithium is less common in the solar system than 25 of the first 32 chemical elements even though the nuclei are very light in atomic weight.[1] For related reasons, lithium has important links to nuclear physics. The transmutation of lithium atoms to helium in 1932 was the first fully man-made nuclear reaction, and lithium-6 deuteride serves as a fusion fuel in staged thermonuclear weapons.[2] Lithium and its compounds have several industrial applications, including heat-resistant glass and ceramics, high strength-to-weight alloys used in aircraft, lithium batteries and lithium-ion batteries. These uses consume more than half of lithium production. Properties[edit] Atomic and physical[edit] Lithium metal is soft enough to be cut with a knife.
Lithium floating in oil. Hydrogen. Hydrogen gas was first artificially produced in the early 16th century, via the mixing of metals with acids.
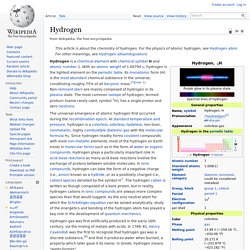
In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance,[8] and that it produces water when burned, a property which later gave it its name: in Greek, hydrogen means "water-former". Industrial production is mainly from the steam reforming of natural gas, and less often from more energy-intensive hydrogen production methods like the electrolysis of water.[9] Most hydrogen is employed near its production site, with the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market.
Hydrogen is a concern in metallurgy as it can embrittle many metals,[10] complicating the design of pipelines and storage tanks.[11] Properties Combustion 2 H2(g) + O2(g) → 2 H2O(l) + 572 kJ (286 kJ/mol)[note 2] H2 reacts with every oxidizing element. Electron energy levels Main article: Hydrogen atom Phases.