

Antidepressant. Serotonin–norepinephrine reuptake inhibitor. Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant drugs used in the treatment of major depression and other mood disorders.
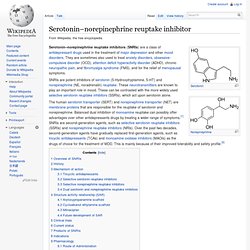
They are sometimes also used to treat anxiety disorders, obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), chronic neuropathic pain, and fibromyalgia syndrome (FMS), and for the relief of menopausal symptoms. SNRIs are potent inhibitors of serotonin (5-Hydroxytryptamine, 5-HT) and norepinephrine (NE, noradrenalin) reuptake. These neurotransmitters are known to play an important role in mood. These can be contrasted with the more widely used selective serotonin reuptake inhibitors (SSRIs), which act upon serotonin alone.
Venlafaxine. Medical uses[edit] Venlafaxine is used primarily for the treatment of depression, general anxiety disorder, social phobia, panic disorder and vasomotor symptoms.[7] At low doses (<150 mg/day), it acts only on serotonergic transmission.
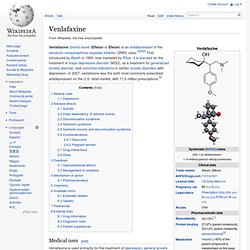
At moderate doses (>150 mg/day), it acts on serotonergic and noradrenergic systems, whereas at high doses (>300 mg/day), it also affects dopaminergic neurotransmission.[8] Many doctors are starting to prescribe venlafaxine "off label" for the treatment of diabetic neuropathy (in a similar manner to duloxetine) and migraine prophylaxis (in some people, however, venlafaxine can exacerbate or cause migraines). Studies have shown venlafaxine's effectiveness for these conditions.[9][10] It has also been found to reduce the severity of 'hot flushes' in menopausal women and men on hormonal therapy for the treatment of prostate cancer.[11][12] Noradrenergic and specific serotonergic antidepressant.
Chemical structure of the prototypical NaSSA mirtazapine (Remeron).
List of NaSSAs[edit] The NaSSAs include the following agents: Aptazapine (CGS-7525A)Esmirtazapine (ORG-50,081)Mianserin (Bolvidon, Norval, Tolvon)[4]Mirtazapine (Remeron, Avanza, Zispin)[4]Setiptiline (Tecipul) Notably, all of these compounds are analogues and are also classified as tetracyclic antidepressants (TeCAs) based on their chemical structures. Recently, S32212, a structurally novel NaSSA with an improved selectivity profile (e.g., no antihistamine effects, etc.), has been developed.[5][6] It has completed preliminary preclinical research and may go on to undergo clinical trials.
Mirtazapine. Mirtazapine (brand names: Avanza, Axit, Mirtaz, Mirtazon, Remeron, Zispin)[6] is a noradrenergic and specific serotonergic antidepressant (NaSSA) introduced by Organon International in the United States in 1996,[2] and is used primarily in the treatment of depression.
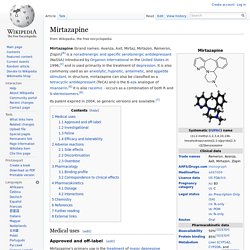
It is also commonly used as an anxiolytic, hypnotic, antiemetic, and appetite stimulant. In structure, mirtazapine can also be classified as a tetracyclic antidepressant (TeCA) and is the 6-aza analogue of mianserin.[6] It is also racemic - occurs as a combination of both R and S-stereoisomers.[6] Its patent expired in 2004, so generic versions are available.[7]
Mianserin. Mianserin Mianserin (brand names: Depnon (IN), Lantanon (ZA), Lerivon (AR, BE, CZ, PL, RU, SK), Lumin (AU), Norval (UK), Tolvon (AU, HK†, IE†, NZ, SG†), Tolmin (DK); where † indicates discontinued products) is a psychoactive drug of the tetracyclic antidepressant (TeCA) therapeutic family.
It is classified as a noradrenergic and specific serotonergic antidepressant (NaSSA) and has antidepressant, anxiolytic (anti-anxiety), hypnotic (sedating), antiemetic (nausea and vomiting-attenuating), orexigenic (appetite-stimulating), and antihistamine effects. It is not approved for use in the US, but its analogue, mirtazapine, is. Mianserin was the first antidepressant to reach the UK market that was less dangerous than the tricyclic antidepressants in overdose.[3] Milnacipran. Milnacipran (Ixel, Savella, Dalcipran, Toledomin) is a serotonin–norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia.
It is not approved for the clinical treatment of major depressive disorder in the USA, but it is in other countries. Medical uses[edit] Depression[edit] In a pooled analysis of 7 comparative trials with imipramine,[1] milnacipran and imipramine were shown to have comparable efficacy while milnacipran was significantly better tolerated. A pooled analysis of studies comparing milnacipran and SSRIs [2] concluded a superior efficacy for milnacipran with similar tolerability for milnacipran and SSRIs. Duloxetine. Duloxetine (sold under the brand names Cymbalta, Ariclaim, Xeristar, Yentreve, Duzela, Dulane) is a serotonin-norepinephrine reuptake inhibitor (SNRI) manufactured and marketed by Eli Lilly.
It is prescribed for major depressive disorder and generalized anxiety disorder (GAD). Duloxetine also has approval for use in osteoarthiritis and musculoskeletal pain. Duloxetine failed the US approval for stress urinary incontinence amidst concerns over liver toxicity and suicidal events; however, it was approved for this indication in Europe, where it is recommended as an add-on medication in stress urinary incontinence instead of surgery.[1] It can also relieve the symptoms of painful peripheral neuropathy, particularly diabetic neuropathy,[2][3] and it is used to control the symptoms of fibromyalgia.
Medical uses[edit] Stress urinary incontinence[edit] Diabetic peripheral neuropathy[edit] Duloxetine was not effective for the numbness or tingling, nor for the other complications of diabetes. Desvenlafaxine. Desvenlafaxine (brand name: Pristiq), also known as O-desmethylvenlafaxine, is an antidepressant of the serotonin-norepinephrine reuptake inhibitor class developed and marketed by Wyeth (now part of Pfizer).
Desvenlafaxine is a synthetic form of the major active metabolite of venlafaxine (sold under the brand names Effexor and Efexor). It is being targeted as the first non-hormonal based treatment for menopause.[1] Norepinephrine-dopamine reuptake inhibitor. A norepinephrine-dopamine reuptake inhibitor (NDRI) is a drug that acts as a reuptake inhibitor for the neurotransmitters norepinephrine and dopamine by blocking the action of the norepinephrine transporter (NET) and the dopamine transporter (DAT), respectively.[1] This in turn leads to increased extracellular concentrations of both norepinephrine and dopamine and, therefore, an increase in adrenergic and dopaminergic neurotransmission.[1] Uses[edit] Norepinephrine-dopamine reuptake inhibitors are used for Clinical depression, ADD, ADHD, Narcolepsy, and as antiparkinson agents.
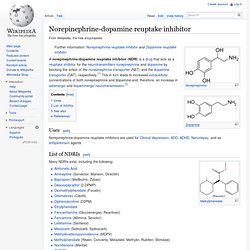
List of NDRIs[edit] Many NDRIs exist, including the following: Note: Only NDRIs selective for the NET and DAT over the serotonin transporter (SERT) are listed here. Bupropion. Bupropion (/bjuːˈproʊpi.ɒn/ bew-PROH-pee-on) (BAN) or bupropion hydrochloride (USAN, BAN), also known as amfebutamone (INN), is a drug of the aminoketone family primarily used as an antidepressant and smoking cessation aid.[8][9][10] Marketed as Wellbutrin and other trade names, it is one of the most frequently prescribed antidepressants in the United States,[11] although in many English-speaking countries, including the United Kingdom, Australia and New Zealand, this is an off-label use.[12] It is also widely used, in a formulation marketed as Zyban, to aid people who are trying to quit smoking.[11] It is taken in the form of tablets, and in the United States and most other countries it is available only with a prescription.[11] Bupropion was synthesized by Nariman Mehta and patented by Burroughs Wellcome in 1969, which later became part of what is now GlaxoSmithKline.
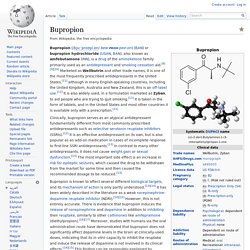
It was first approved for clinical use in the United States in 1989. Selective serotonin reuptake inhibitor. Selective serotonin re-uptake inhibitors or serotonin-specific reuptake inhibitor[1] (SSRIs) are a class of compounds typically used as antidepressants in the treatment of depression, anxiety disorders, and some personality disorders. SSRIs are believed to increase the extracellular level of the neurotransmitter serotonin by inhibiting its reuptake into the presynaptic cell, increasing the level of serotonin in the synaptic cleft available to bind to the postsynaptic receptor.
They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having only weak affinity for the noradrenaline and dopamine transporter. Sertraline. Sertraline (trade names Zoloft, Lustral) is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It was introduced to the market by Pfizer in 1991. Paroxetine. Fluvoxamine. Fluvoxamine (brand names: Floxyfral, Luvox, Fevarin) is a medication which functions as a selective serotonin reuptake inhibitor (SSRI) and σ1 receptor agonist. Fluvoxamine is used primarily for the treatment of obsessive-compulsive disorder (OCD),[3] and is also used to treat major depressive disorder (MDD), and anxiety disorders such as panic disorder and posttraumatic stress disorder (PTSD).[4] Fluvoxamine CR (controlled release) is approved to treat social anxiety disorder.[5] The FDA has added a black box warning for this drug in reference to increased risks of suicidal thinking and behavior in young adults and children.
A study from the Institute for Safe Medication Practices identified reports of violence from those taking fluvoxamine as being 8.4 times higher than expected given the volume of overall reports for that drug. Fluoxetine. Fluoxetine (also known by the tradenames Prozac, Sarafem, Ladose and Fontex, among others) is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Fluoxetine was first documented in 1974 by scientists from Eli Lilly and Company.[6] It was approved by the U.S. Food and Drug Administration for the treatment of major depressive disorder in December 1987.[7] The fluoxetine patent expired in August 2001.[8] Despite the availability of newer agents, fluoxetine remains extremely popular.
In 2010, over 24.4 million prescriptions for generic formulations of fluoxetine were filled in the United States alone,[11] making it the third most prescribed antidepressant after sertraline (SSRI; became generic in 2006) and citalopram (SSRI; became generic in 2003).[11] In 2011, 6 million prescriptions for fluoxetine were handed out in the UK.[12] Medical uses[edit] Escitalopram. Citalopram. Citalopram (/sɪˈtælɵpræm/ or /saɪˈtælɵpræm/; brand names: Celexa, Cipramil) is an antidepressant drug of the selective serotonin reuptake inhibitor (SSRI) class. Monoamine oxidase inhibitor. Monoamine oxidase inhibitors (MAOIs) are chemicals which inhibit the activity of the monoamine oxidase enzyme family. Isocarboxazid. Moclobemide. Phenelzine. Selegiline. Tranylcypromine. Tricyclic antidepressant. Amitriptyline. Clomipramine. Clomipramine (trademarked as Anafranil) is a tricyclic antidepressant (TCA). It was developed in the early 1960s[2] by the Swiss drug manufacturer Geigy (now known as Novartis) and has been in clinical use worldwide ever since.
It is the 3-chlorinated derivative of the earlier tricyclic, imipramine. Imipramine. Trimipramine. Doxepin. Norepinephrine-dopamine disinhibitor. Agomelatine. Selective serotonin reuptake enhancer. Tianeptine. Norepinephrine reuptake inhibitor. Atomoxetine. Mazindol. Reboxetine. Viloxazine.