

Rare-earth metal recycling needed to power green tech. Metal recycling is the sleeper growth industry in green tech.
Specialty metals, such as lithium and indium, and rare-earth elements, such as neodymium, are required for production of many green-technology products, including batteries for hybrid cars, LED lights, fuel cells, and solar panels. But to ensure future supply of these resources, recycling rates needed to increase substantially, according to a report from the United Nations Environment Program. Preliminary findings were issued Thursday, with a full report planned for later this year. The recycling rates for specialty metals are only about 1 percent, according to a U.N. panel on metals that is chaired by experts from India, Germany, and Yale University. There are substantial environmental benefits to recycling all metals, which is between two and ten times more energy efficient than smelting metals from ores, according to the report. Lyc.
R41347. Rare Earth Elements Stocks - Pele Mountain Company - Stock Symbol GEM: TSX-V. Rare Earth minerals have been hitting the financial headlines recently - Panel discussion. Rare Earth Mining Stocks in a Bubble. Rare Earth Elements. China's Rare Earth Dominance. Why The Chinese Monopoly On Rare Earth Elements Is So Incredibly Dangerous. Most Americans have no idea why rare earth elements are important or why the Chinese monopoly on them is so dangerous.
But now that China is enforcing strict new quotas on the export of these metals a lot more people are going to start learning about them. So just exactly what are they? Well, “rare earth elements” is a name that has been given to 17 metals from the middle of the Periodic Table with nearly unpronounceable names such as lanthanum, cerium, tantalum, neodymium and europium. These metals are used in an increasing number of high technology products. Everything from iPods to wind turbines to missile-guidance systems use these metals. It was a huge mistake for the rest of the world to let China develop such a monopoly. Russia May Challenge China’s Rare Earth Dominance. By Michael Montgomery-Exclusive to Rare Earth Investing News The dominance of China over the rare earth market is well documented.
The country controls over 95 percent of the world supply. However, due to changes in trade policy, the world is actively seeking out new deposits to secure supply of these vital elements. The Chinese stated earlier in the month that ' drastic ' changes to their rare earth industry are coming soon, and even reports that the country will keep more of their production for a strategic stockpile. The cuts to exports and subsequent price increases helped China increase the value of their exports in January by 376 percent "despite a decline in volume of 29 percent from January of last year," reported the International Business Times .
The potential for even deeper cuts to exports loom over the entire industry. Rare earth mines in Russia that were not planned to be developed until 2030 are quickly becoming more enticing. Yekaterinburg companies. METALRESEARCH - METALRESEARCH: Russian rare-earth metals market 2010 - InterStol Russia. Rare earth element. As defined by IUPAC, a rare earth element (REE) or rare earth metal is one of a set of seventeen chemical elements in the periodic table, specifically the fifteen lanthanides, as well as scandium and yttrium.[2] Scandium and yttrium are considered rare earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties.
List[edit] A table listing the seventeen rare earth elements, their atomic number and symbol, the etymology of their names, and their main usages (see also Applications of lanthanides) is provided here. Some of the rare earth elements are named after the scientists who discovered or elucidated their elemental properties, and some after their geographical discovery. A mnemonic for the names of the sixth-row elements in order is "Lately college parties never produce sexy European girls that drink heavily even though you look".[6] Abbreviations[edit] The following abbreviations are often used:
Scandium. Scandium is a chemical element with symbol Sc and atomic number 21.
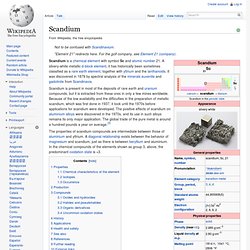
A silvery-white metallic d-block element, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia. Scandium is present in most of the deposits of rare earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide.
Because of the low availability and the difficulties in the preparation of metallic scandium, which was first done in 1937, it took until the 1970s before applications for scandium were developed. The positive effects of scandium on aluminium alloys were discovered in the 1970s, and its use in such alloys remains its only major application. Yttrium. Yttrium is a chemical element with symbol Y and atomic number 39.
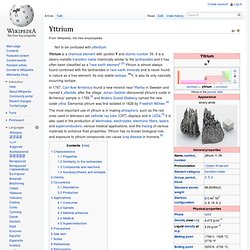
It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element".[2] Yttrium is almost always found combined with the lanthanides in rare earth minerals and is never found in nature as a free element. Its only stable isotope, 89Y, is also its only naturally occurring isotope. In 1787, Carl Axel Arrhenius found a new mineral near Ytterby in Sweden and named it ytterbite, after the village. Johan Gadolin discovered yttrium's oxide in Arrhenius' sample in 1789,[3] and Anders Gustaf Ekeberg named the new oxide yttria. Lanthanum. Lanthanum is a chemical element with the symbol La and atomic number 57.
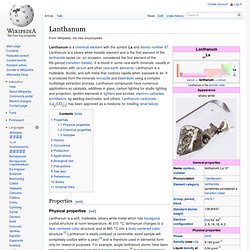
Lanthanum is a silvery white metallic element and is the first element of the lanthanide series (or, on occasion, considered the first element of the 6th-period transition metals). It is found in some rare-earth minerals, usually in combination with cerium and other rare earth elements. Lanthanum is a malleable, ductile, and soft metal that oxidizes rapidly when exposed to air. It is produced from the minerals monazite and bastnäsite using a complex multistage extraction process. Cerium. Characteristics[edit] Physical properties[edit] Cerium is a silvery metal, belonging to the lanthanide group.
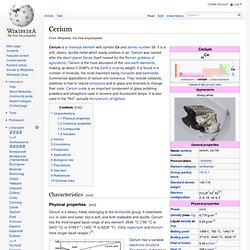
Rare%2BEarth%2BElements%2Bcomparison%2Bphoto. Praseodymium. Praseodymium is a chemical element that has the symbol Pr and atomic number 59.
Praseodymium is a soft, silvery, malleable and ductile metal in the lanthanide group. Valued for its magnetic, electrical, chemical and optical properties,[3] it is too reactive to be found in native form, and when artificially prepared, it slowly develops a green oxide coating. The element was named for the color of its primary oxide. In 1841, Swedish chemist Carl Gustav Mosander extracted a rare earth oxide residue he called "didymium" from a residue he called "lantana," in turn separated from cerium salts.
In 1885, the Austrian chemist Baron Carl Auer von Welsbach separated didymium into two salts of different colors, which he named praseodymium and neodymium. Like most rare earth elements, praseodymium most readily forms trivalent Pr(III) ions. Characteristics[edit] Physical properties[edit] Neodymium. Neodymium compounds were first commercially used as glass dyes in 1927, and they remain a popular additive in glasses.
The color of neodymium compounds—due to the Nd3+ ion—is often a reddish-purple but it changes with the type of lighting, due to fluorescent effects. Some neodymium-doped glasses are also used in lasers that emit infrared light with wavelengths between 1047 and 1062 nanometers. These have been used in extremely high power applications, such as experiments in inertial confinement fusion. Another chief use of neodymium is as the free pure element. Promethium. In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was no element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both "discoveries" were soon proven to be false.
In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor.
Properties[edit] Physical properties[edit] Samarium. Samarium was discovered in 1879 by the French chemist Paul Émile Lecoq de Boisbaudran and named after the mineral samarskite from which it was isolated. The mineral itself was earlier named after a Russian mine official, Colonel Vasili Samarsky-Bykhovets, who thereby became the first person to have a chemical element named after him, albeit indirectly.