

Foam Bonding Techniques Part 1. Plastipedia: Plastics Processes. Login Join the BPF Join the BPF Mailing List Related Categories Plastipedia Plastics Processes.
Vinyl chloride. Vinyl chloride is an organochloride with the formula H2C=CHCl that is also called vinyl chloride monomer, VCM or chloroethene.
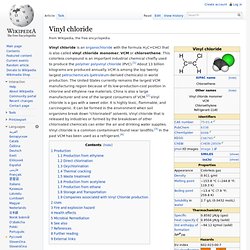
This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC).[1] About 13 billion kilograms are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM.[2] Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. Production[edit] Polyvinyl chloride. Poly(vinyl chloride), commonly abbreviated PVC, is the third-most widely produced polymer, after polyethylene and polypropylene.[4] PVC is used in construction because it is more effective than traditional materials such as copper, iron or wood in pipe and profile applications.
It can be made softer and more flexible by the addition of plasticizers, the most widely used being phthalates. In this form, it is also used in plumbing, electrical cable insulation, inflatable products and many applications where it replaces rubber.[5] Polypropylene. Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging and labeling, textiles (e.g., ropes, thermal underwear and carpets), stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes.
An addition polymer made from the monomer propylene, it is rugged and unusually resistant to many chemical solvents, bases and acids. In 2008, the global market for polypropylene had a volume of 45.1 million metric tons, which led to a turnover of about $65 billion (~ €47.4 billion).[1] Chemical and physical properties[edit] Degradation[edit]
Coal tar. Applications[edit] Pavement sealcoat[edit] Coal tar is incorporated into some parking-lot sealcoat products, which are used to protect and beautify the underlying pavement.[2] Sealcoat products that are coal-tar based typically contain 20 to 35 percent coal-tar pitch.[2] Substantial concerns have been raised about the safety of this application of coal tar, given that coal tar is known to cause cancer in humans and that several PAH compounds in coal tar are toxic to aquatic life.[3] The primary use of coal tar based sealcoats is regional within the US[2] but federal research [4] shows it is used in states from Alaska to Florida and several areas have banned its use in sealcoat products [5][6][7] including: The District of Columbia; the City of Austin, Texas; Dane County, Wisconsin; Washington State; and several municipalities in Minnesota and others.[8][9] Industrial[edit]
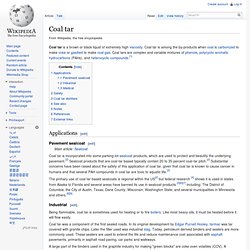
Naphthalene. Naphthalene is an organic compound with formula C 10H 8.
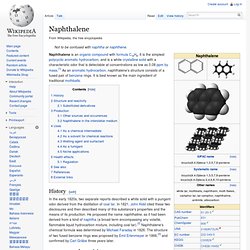
It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass.[1] As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs. History[edit] Structure and reactivity[edit] Phthalic anhydride. Phthalic anhydride is the organic compound with the formula C6H4(CO)2O.
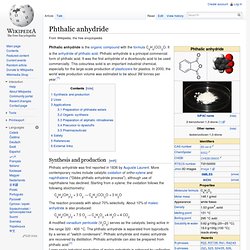
It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This colourless solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the world wide production volume was estimated to be about 3M tonnes per year.[1] Synthesis and production[edit] Phthalic acid. Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2.
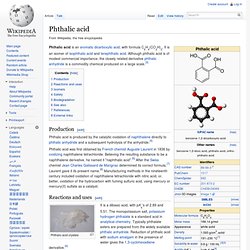
It is an isomer of isophthalic acid and terephthalic acid. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale.[5] Production[edit] Phthalic acid is produced by the catalytic oxidation of naphthalene directly to phthalic anhydride and a subsequent hydrolysis of the anhydride.[5] Terephthalic acid. Terephthalic acid is the organic compound with formula C6H4(COOH)2.
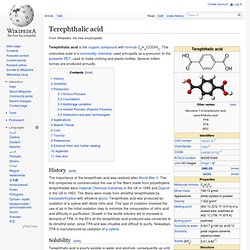
This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced annually. History[edit] Plasticizer. For plastics[edit] Plasticizers for plastics are additives, most commonly phthalate esters in PVC applications.
Almost 90% of the market for plasticizer is for PVC, giving this material improved flexibility and durability.[1] The majority is used in films and cables.[2] Plasticizers work by embedding themselves between the chains of polymers, spacing them apart (increasing the "free volume"), and thus significantly lowering the glass transition temperature for the plastic and making it softer. For plastics such as PVC, the more plasticizer added, the lower its cold flex temperature will be. This means that it will be more flexible and its durability will increase as a result of it. Plasticizers evaporate and tend to concentrate in an enclosed space; the "new car smell" is caused mostly by plasticizers evaporating from the car interior.
Plasticizers make it possible to achieve improved compound processing characteristics, while also providing flexibility in the end-use product. Petroleum. An oil refinery in Mina-Al-Ahmadi, Kuwait The use of fossil fuels such as petroleum has a negative impact on Earth's biosphere, releasing pollutants and greenhouse gases into the air and damaging ecosystems through events such as oil spills.
Concern over the depletion of the earth's finite reserves of oil, and the effect this would have on a society dependent on it, is a concept known as peak oil. Etymology[edit] The word petroleum comes from Greek: πέτρα (petra) for rocks and Greek: ἔλαιον (elaion) for oil. History[edit] Early history[edit] Naphtha.
Viscoelastic Phase Separation in Epoxy/Thermoplastic Blends. Thermosetting resins are usually low molecular weight monomers or oligomers having functional groups for cross-linking reaction. The polymerization of these resins (curing reaction) can be carried out either by addition reaction, without evolution of volatiles (e.g., epoxy resin, unsaturated polyester, or addition polyimides) or by condensation reaction (e.g., phenolic resin) to yield a highly cross-linked three-dimensional network structure which is infusible and insoluble in most of the organic solvents. Transesterification. Transesterification: alcohol + ester → different alcohol + different ester Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to drive equilibrium.
Polyester. SEM picture of a bend in a high-surface area polyesterfiber with a seven-lobed cross section Close-up of a polyester shirt Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate (PET).
Polyesters include naturally occurring chemicals, such as in the cutin of plant cuticles, as well as synthetics through step-growth polymerization such as polycarbonate and polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Depending on the chemical structure, polyester can be a thermoplastic or thermoset, there are also polyester resins cured by hardeners; however, the most common polyesters are thermoplastics.[1]
Exotic discovery made in soft polymer. Professor Frank S. Bates and his research team at the University of Minnesota in Minneapolis have discovered an unusual type of soft material that was conceived of over 50 years ago, but has never before been found in a plastic--although it has been seen in stainless steel and other metal alloys. Bates' group, which is funded by the National Science Foundation's Division of Materials Research, specializes in creating a particular class of material, called block copolymers.
Scientists and engineers create the 'perfect plastic' (10/7/2011) Researchers at the University of Leeds and Durham University have solved a long-standing problem that could revolutionize the way new plastics are developed. Solving a tangled polymer problem. New elastic material changes color in UV light. Researchers from North Carolina State University have created a range of soft, elastic gels that change color when exposed to ultraviolet (UV) light -- and change back when the UV light is removed or the material is heated up. Frameset Page.
Polyhydroxyalkanoates. Polymer. Polymer Physics. Polymer. IUPAC definition Substance composed of macromolecules. Note: Applicable to substance macromolecular in nature like cross-linked systems that can be considered as one macromolecule. The term "polymer" derives from the ancient Greek word πολύς (polus, meaning "many, much") and μέρος (meros, meaning "parts"), and refers to a molecule whose structure is composed of multiple repeating units, from which originates a characteristic of high relative molecular mass and attendant properties.[6] The units composing polymers derive, actually or conceptually, from molecules of low relative molecular mass.[7] The term was coined in 1833 by Jöns Jacob Berzelius, though with a definition distinct from the modern IUPAC definition.[8][9] The modern concept of polymers as covalently bonded macromolecular structures was proposed in 1920 by Hermann Staudinger, who spent the next decade finding experimental evidence for this hypothesis.[10] Common examples[edit]
The Plastics Historical Society - What are Plastics? The Early Years Of Artificial Fibres. Plastipedia: The Plastics Encyclopedia - A History of Plastics. The Plastics Historical Society - People & Polymers. Tougher, lighter wind turbine blade developed: Polyurethane reinforced with carbon nanotubes. Efforts to build larger wind turbines able to capture more energy from the air are stymied by the weight of blades. Polymer batteries for next-generation electronics. (PhysOrg.com) -- University of Leeds scientists have invented a new type of polymer gel that can be used to manufacture cheaper lithium batteries without compromising performance. The technology, developed by Professor Ian Ward FRS, a Research Professor of Physics at the University of Leeds, has been licensed to the American company Polystor Energy Corporation, which is conducting trials to commercialise cells for portable consumer electronics.