

Double-slit experiment. The double-slit experiment is a demonstration that light and matter can display characteristics of both classically defined waves and particles; moreover, it displays the fundamentally probabilistic nature of quantum mechanical phenomena.
The experiment belongs to a general class of "double path" experiments, in which a wave is split into two separate waves that later combine back into a single wave. Changes in the path lengths of both waves result in a phase shift, creating an interference pattern. Another version is the Mach–Zehnder interferometer, which splits the beam with a mirror. Thomas Young (scientist) Thomas Young (13 June 1773 – 10 May 1829) was an English polymath.
Young made notable scientific contributions to the fields of vision, light, solid mechanics, energy, physiology, language, musical harmony, and Egyptology. He "made a number of original and insightful innovations"[1] in the decipherment of Egyptian hieroglyphs (specifically the Rosetta Stone) before Jean-François Champollion eventually expanded on his work. He was mentioned by, among others, William Herschel, Hermann von Helmholtz, James Clerk Maxwell, and Albert Einstein. Young belonged to a Quaker family of Milverton, Somerset, where he was born in 1773, the eldest of ten children. Radioactive decay. Alpha decay is one example type of radioactive decay, in which an atomic nucleus emits an alpha particle, and thereby transforms (or 'decays') into an atom with a mass number decreased by 4 and atomic number decreased by 2.
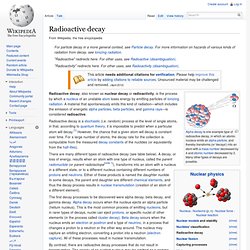
Many other types of decays are possible. Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting particles of ionizing radiation. A material that spontaneously emits this kind of radiation—which includes the emission of energetic alpha particles, beta particles, and gamma rays—is considered radioactive. Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay.[1] However, the chance that a given atom will decay is constant over time.
Henri Becquerel. Antoine Henri Becquerel (15 December 1852 – 25 August 1908) was a French physicist, Nobel laureate, and the discoverer of radioactivity along with Marie Skłodowska-Curie and Pierre Curie,[1] for which all three won the 1903 Nobel Prize in Physics.
Biography[edit] Early life[edit] Becquerel was born in Paris into a family which produced four generations of scientists: Becquerel's grandfather (Antoine César Becquerel), father (Alexandre-Edmond Becquerel), and son (Jean Becquerel). He studied engineering at the École Polytechnique and the École des Ponts et Chaussées. In 1890 he married Louise Désirée Lorieux. Electron. History[edit] In the early 1700s, Francis Hauksbee and French chemist Charles François de Fay independently discovered what they believed were two kinds of frictional electricity—one generated from rubbing glass, the other from rubbing resin.
From this, Du Fay theorized that electricity consists of two electrical fluids, vitreous and resinous, that are separated by friction, and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought of the charge carrier as being positive, but he did not correctly identify which situation was a surplus of the charge carrier, and which situation was a deficit.[19] Discovery[edit] J. J. Thomson. In 1897 Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, and thus he is credited with the discovery and identification of the electron; and, in a broader sense, with the discovery of the first subatomic particle.
Thomson is also credited with finding the first evidence for isotopes of a stable (non-radioactive) element in 1913, as part of his exploration into the composition of canal rays (positive ions). He invented the mass spectrometer. Black-body radiation. As the temperature decreases, the peak of the black-body radiation curve moves to lower intensities and longer wavelengths.
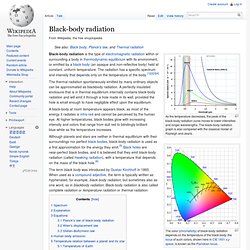
The black-body radiation graph is also compared with the classical model of Rayleigh and Jeans. Black-body radiation is the type of electromagnetic radiation within or surrounding a body in thermodynamic equilibrium with its environment, or emitted by a black body (an opaque and non-reflective body) held at constant, uniform temperature. Photoelectric effect. The photoelectric effect is the observation that many metals emit electrons when light shines upon them.
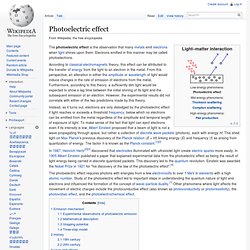
Electrons emitted in this manner may be called photoelectrons. According to classical electromagnetic theory, this effect can be attributed to the transfer of energy from the light to an electron in the metal. Oil drop experiment. Millikan's setup for the oil drop experiment The oil drop experiment was an experiment performed by Robert A.
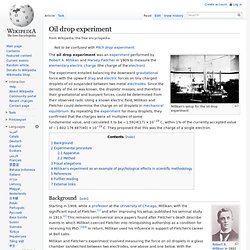
Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment entailed balancing the downward gravitational force with the upward drag and electric forces on tiny charged droplets of oil suspended between two metal electrodes. Since the density of the oil was known, the droplets' masses, and therefore their gravitational and buoyant forces, could be determined from their observed radii. Using a known electric field, Millikan and Fletcher could determine the charge on oil droplets in mechanical equilibrium. Robert Andrews Millikan. Robert A.
Millikan (March 22, 1868 – December 19, 1953) was an American experimental physicist honored with the Nobel Prize for Physics in 1923 for his measurement of the elementary electronic charge and for his work on the photoelectric effect. Millikan graduated from Oberlin College in 1891 and obtained his doctorate at Columbia University in 1895. Geiger–Marsden experiment. The Geiger–Marsden experiment (also called the Rutherford gold foil experiment) was a landmark experiment by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass is concentrated. They deduced this by observing how alpha rays are scattered when they pass through a thin metal foil. Ernest Rutherford. "Lord Rutherford" redirects here; not to be confused with Lord Rutherfurd or with Andrew Rutherford, 1st Earl of Teviot. Ernest Rutherford, 1st Baron Rutherford of Nelson, OM FRS[1] (30 August 1871 – 19 October 1937) was a New Zealand-born British physicist who became known as the father of nuclear physics.[2] Encyclopædia Britannica considers him to be the greatest experimentalist since Michael Faraday (1791–1867).[2]
Franck–Hertz experiment. Photograph of a vacuum tube with a drop of mercury that's used for the Franck–Hertz experiment in instructional laboratories. A - anode disk. G - metal mesh grid. C - cathode assembly; the cathode itself is hot, and glows orange. The Franck–Hertz experiment was the first electrical measurement to clearly show the quantum nature of atoms, and thus "transformed our understanding of the world".[1] It was presented on April 24, 1914 to the German Physical Society in a paper by James Franck and Gustav Hertz.[2][3] Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor of mercury atoms. They discovered that, when an electron collided with a mercury atom, it could lose only a specific quantity of its kinetic energy (7.8×10-19 joules or 4.9 electron volts).
These experimental results proved to be consistent with the Bohr model for atoms that had been proposed the previous year by Niels Bohr. Gustav Ludwig Hertz. Gustav Ludwig Hertz (22 July 1887 – 30 October 1975) was a German experimental physicist and Nobel Prize winner, and a nephew of Heinrich Rudolf Hertz. Biography[edit] Hertz was born in Hamburg, the son of Auguste (née Arning) and a lawyer, Gustav Theodor Hertz (1858–1904),[1] Heinrich Rudolf Hertz' brother.
He studied at the Georg-August University of Göttingen (1906–1907), the Ludwig Maximilians University of Munich (1907–1908), and the Humboldt University of Berlin (1908–1911). He received his doctorate in 1911 under Heinrich Leopold Rubens.[2][3] James Franck. James Franck (26 August 1882 – 21 May 1964) was a German physicist and Nobel laureate.[1][2][3][4] Biography[edit] Franck was Jewish; his parents were Jacob Franck and Rebecca Nachum Drucker. Stern–Gerlach experiment. Basic theory and description[edit] Walter Gerlach. Otto Stern. Electron diffraction. Electron diffraction is most frequently used in solid state physics and chemistry to study the crystal structure of solids. Lester Germer. Clinton Davisson. Clinton Joseph Davisson (October 22, 1881 – February 1, 1958), was an American physicist who won the 1937 Nobel Prize in Physics for his discovery of electron diffraction in the famous Davisson-Germer experiment. Davisson shared the Nobel Prize with George Paget Thomson, who independently discovered electron diffraction at about the same time as Davisson.
Cowan–Reines neutrino experiment. Clyde Cowan. Clyde Lorrain Cowan Jr (December 6, 1919 in Detroit, Michigan - May 24, 1974 in Bethesda, Maryland) was an American physicist, the co-discoverer of the neutrino along with Frederick Reines. Frederick Reines. Frederick Reines (March 16, 1918 – August 26, 1998) was an American physicist. Hall effect. Quantum Hall effect. Klaus von Klitzing. Bell test experiments. Quantum entanglement. Alain Aspect. Mach–Zehnder interferometer. Elitzur–Vaidman bomb tester. Interaction-free measurement.